| |
| Clin Pharmacol Ther. 2012 May; 91(5): 777–86. doi: 10.1038/clpt.2011.309.Pharmacokinetic and pharmacodynamic variability of fluindione in octogenarians Emmanuelle Comets,1* Bertrand Diquet,2 Sylvie Legrain,3 Marie-Geneviève Huisse,4 Alban Godon,5 Corinne Bruhat,6 Marie-Paule Chauveheid,7 Sandrine Delpierre,3 Xavier Duval,1,8 Gilles Berrut,9 Céline Verstuyft,10,11 Marie-Claude Aumont,12 and France Mentré1 1Modèles et Méthodes de l'Evaluation Thérapeutique des Maladies Chroniques
, INSERM : U738, Faculté de Médecine - Université Paris VII - Paris Diderot, 16, Rue Henri Huchard 75018 Paris, FR 2Département de Biologie des Agents Infectieux et Pharmacotoxicologie
, CHU Angers, PRES Université Nantes Angers Le Mans (UNAM), 4 Rue Larrey 49100 Angers, FR 3Service de Gériatrie [Bretonneau]
, Hôpital Bretonneau, Assistance publique - Hôpitaux de Paris (AP-HP), Université Paris VII - Paris Diderot, 23 Rue Joseph de Maistre 75018 Paris, FR 4Service d'Hématologie et Immunologie
, Hôpital Bichat - Claude Bernard, Assistance publique - Hôpitaux de Paris (AP-HP), Université Paris VII - Paris Diderot, 46 Rue Henri Huchard 75018 Paris, FR 5Service d'Hématologie [Angers]
, CHU Angers, 4 Rue Larrey 49100 Angers, FR 6Service de Gériatrie [Angers]
, CHU Angers, 4 Rue Larrey 49100 Angers, FR 7Service de Médecine Interne [Bichat]
, Hôpital Bichat - Claude Bernard, Assistance publique - Hôpitaux de Paris (AP-HP), 46 Rue Henri Huchard 75018 Paris, FR 8CIC - CHU Bichat
, INSERM : CIC7, Hôpital Bichat - Claude Bernard, Assistance publique - Hôpitaux de Paris (AP-HP), 46, Rue Henri Huchard 75018 Paris, FR 9Pôle de Gérontologie
, Université de Nantes, 2 Chemin de la Houssinière 44300 Nantes, FR 10Service de Génétique Moléculaire, Pharmacogénétique et Hormonologie
, Hôpital Bicêtre, Assistance publique - Hôpitaux de Paris (AP-HP), Université Paris XI - Paris Sud, 78, rue du Général Leclerc 94275 Le Kremlin Bicêtre, FR 11Barrières Physiologiques et Réponses Thérapeutiques
, Université Paris XI - Paris Sud : EA4123 - UFR de Pharmacie, Bâtiment D1 - 92296 Chatenay-Malabry, FR 12Service de Cardiologie
, Hôpital Bichat - Claude Bernard, Assistance publique - Hôpitaux de Paris (AP-HP), Université Paris VII - Paris Diderot, 46, Rue Henri Huchard 75018 Paris, FR |
In the PREPA observational study, we investigated the factors influencing pharmacokinetic and pharmacodynamic variability in the response to fluindione, an oral anticoagulant drug, in a general population of octogenarians inpatients. Measurements of fluindione concentrations and INR (International Normalised Ratio) were obtained from 131 inpatients initiating fluindione treatment. Treatment was adjusted according to routine clinical practice. The data was analysed using non-linear mixed effect models, and the parameters were estimated using MONOLIX 3.2. The pharmacokinetics of fluindione was monocompartmental, while the evolution of INR was modelled according to a turnover model (inhibition of vitamin K recycling). Interindividual variability was very large. Clearance decreased with age and with prior administration of cordarone. Patients who underwent surgery before the study had lower IC50, leading to an increased sensitivity to fluindione. Pharmacokinetic exposure is substantially increased in elderly patients, warranting a lower dose of fluindione. MeSH keywords: Aged, Aged, 80 and over, Anticoagulants, pharmacokinetics, pharmacology, Female, Humans, International Normalized Ratio, Male, Phenindione, analogs & derivatives, pharmacokinetics, pharmacology Author keywords: fluindione, antivitamin K, oral anticoagulant, elderly, pharmacokinetics, pharmacodynamics, International Normalised Ratio (INR) |
Evidence-based clinical practice guidelines recommend vitamin K antagonists in the prevention of stroke in atrial fibrillation [1, 2], in the treatment of venous thromboembolism [3], in patients with mechanical valve and in the first 3 months following bioprotheses implantation [4]. Despite their proven benefit, studies attest to their underutilisation particularly among elderly individuals [5, 6]. Antivitamin K agents (AVK) act by inhibiting the reduction reactions by which the vitamin K is recycled, in turn decreasing the synthesis of vitamine K-dependent coagulation factors (factors II (prothrombin), VII, IX, X, protein C, and protein S) [7]. The two main classes are coumarin derivatives, including warfarin and acenocoumarol, and indanedione derivatives, including fluindione and phenindione. Fluindione constitutes about 80% of AVK prescriptions in France [8]. AVK are characterised by a large between-, but also within-patient variability in the dose-response relationship. The therapeutic window is narrow, so that clinicians must walk a thin line between suboptimal dosage risking thromboembolic events and higher doses potentially risking bleeding episodes. The frequency and seriousness of haemorragic adverse events varies in the literature, depending on the population, the prescribed therapeutic range, the other treatments co-administered and the duration of treatment with AVK [9]. The Adverse Event Reporting system of the US Food and Drug Administration provides evidence that warfarin is among the top 10 drugs with the greatest number of serious adverse events. In France, where the present study was performed, iatrogenic events due to oral anticoagulant drugs represent the first cause of hospital admission for drug-related adverse event [10], totalling about 17000 admissions a year, and an estimated 3000 to 5000 deaths [11]. A meta-analysis of available clinical trials in patients anticoagulated for venous thromboembolism, reported a case-fatality rate of major bleeding of 13.4% in all patients (95% confidence interval 9.4 to 17.4%) [12]. Haemorragic events are overall more frequent and more severe in elderly patients compared to the general population [13]. The contribution of age per se to this increased risk is somewhat controversial, some studies pointing to an increase of the incidence of haemorragic events as a function of age [9] while others do not find it significant [14], but the fact that severity increases with age is undisputed [15] so that scores developed to predict the risk of bleeding include age over 65 as an independent risk factor [2, 16]. The main risk factors known to bring about haemorragic complications are level of anticoagulation, poor quality of monitoring, lack of patient eduction, associated comorbidities and comedications, including interactions with drugs interfering with haemostasis, and being in the first months of treatment [9, 14, 17]. Variability in International Normalised Ratio (INR) levels is also higher in elderly patients [18, 19]. A large part of this variability can be explained by changes in the dose-concentration relationship (pharmacokinetics, PK), or in the concentration-response relationship (pharmacodynamics, PD). Measurement of AVK concentrations can contribute to a better understanding of these two components by separating these two contributions. In the present paper, we describe the findings from the PREPA study, investigating the pharmacokinetics and pharmacodynamics of fluindione in octogenarians using non-linear mixed effect models. The primary objective of PREPA was to study the factors influencing the source of variability in the response to fluindione in elderly inpatients starting fluindione, with a special interest in comorbidities and comedications. |
2.1. Data 151 subjects were recruited in PREPA, 131 of whom provided PK/PD data and were included in the present PK/PD analysis. Table 1 shows the demographic and biologic variables recorded in this population. The prescribed therapeutic range for INR was [2–3] for all the patients included in the study. The elderly patients included in the PREPA study were generally polymedicated: on the first day of the study, they received on average 8 different medications in addition to fluindione. In this study, initial dosing of fluindione was conservative: the initial dose was 5, 10 and 15–20 mg in 28, 94 and 10 subjects respectively. The median duration of stay in the study was 8 days (range 2 to 31 days). The last dose of fluindione was 5 mg or less for 32 subjects, 7.5–10 mg for 54 patients, 12.5–15 mg for 30 patients and 17.5–22.5 mg for 15 patients. There were large variations between the initial and final dose (correlation 0.25), with the dose unchanged in 44 subjects (34%) while 32 had a lower dose (24%) and 55 a higher dose (42%). This study was not designed to evaluate a maintenance dose of fluindione, and only 52 patients (40%) left the study with an INR between 2 and 3. Ten subjects (8%) experienced bleeding during the study, as described elsewhere [20]. Nine of these patients also received heparin prior to initiating fluindione treatment, and severe bleeding occurred in 5 of them, always associated with the heparin treatment. The tenth subject, who suffered from hemorroids and constipation, started heparin on day 2 and experienced minor bleeding the next day, which resolved quickly. 2.2. Base model building The PK dataset included 493 concentrations of fluindione, and the PD dataset 477 measurements of INR. A one-compartment model without lag-time provided an adequate fit to the PK data, based on tests and diagnostic graphs. Although the estimation error for the absorption rate constant k a was reported as reasonable, its interindividual variability (IIV) was large, and this parameter proved relatively unstable from run to run, especially at later stages when covariates were included in the model. Because there was little information about the absorption phase, we considered a model where k a was fixed without variability. In a study in healthy volunteers, fluindione was found to be quickly absorbed, with an average T max of 2.0 h (range 0.5–6.0 h) [ 21], while the elimination half-life was 35 h (SD 6.5 h). Based on these figures, we fixed k a to 2.42 hr −1. This improved model stability, and provided similar estimates of V and CL. IIV was estimated for CL and V, without covariance. An indirect response model for 1/INR was found adequate in the PK/PD analysis. INR values increase from baseline value of 1 in a normal patient, and the estimate of the additive part of the combined error model converged to a very small value. Therefore the residual variability for the INR model was modelled as proportional. A diagonal covariance matrix was used to model IIV. The Hill coefficient was significantly different from 1, and assuming a linear model instead of the Imax model also degraded the fit. Models including precursors were also tested to account for time delays but did not improve the fit or the likelihood. 2.3. Covariate model building Covariates were first included on V and CL. Using the individual parameters estimates from the base model, the following covariates were found to have an influence on V, CL or both, and were considered for inclusion in the model: gender, weight, age, surgery, atrial fibrillation, renal function, Mini-Mental State (MMS) score, as well as administration of cordarone and deroxat. After pruning down the model, the following relationships remained: the volume of distribution V was found to increase with weight, and to be higher in men; clearance CL on the other hand was found to decrease with age, and to be lower in patients who received cordarone during the study. We also explored the relationships between parameters and time-varying comedications by considering each occasion as a separate subject. None of these comedications was found to influence the two PK parameters. For the PD parameters, the following candidate relationships were found: CLCR, nonagerian, surgery and protamine on I0, deroxat on Imax, CLCR, surgery and protamine on kout, nonagerian, surgery and protamine on C50, Activities of Daily Living (ADL) score, surgery and deroxat on γ. In the final model, patients recovering from cardiac surgery were found to have reduced C50 and γ, translating to higher sensitivity. These patients were younger but age did not remain in the model. Deroxat increased γ. The variability on Imax was poorly estimated and was removed from the final model. In most models we found I0 to be very close to 1, but the assumption I0=1 led to a significant increase in the statistical criterion. 2.4. Final model
Table 2 shows the parameter estimates for the base and final model (the range obtained by multiple imputation is given in Supplementary Table S2, showing the robustness of the estimates). There was a small decrease in the estimates of the variabilities of all parameters except k out when including covariates in the model, however the IIV remained large. Relative standard errors were less than 10% for the main parameters, and within a 10–30% range for the variability of the random effects. Compared to women, men had an apparent volume of distribution increased by about 25%. The increase of V with weight was relatively small, since an increase of 10 kg in weight translates to about 9% increase in V. The decrease of CL with age is more relevant, since we expect a 90 year old patient to have a clearance reduced by 30% compared to a 80 year old patient, and patients who received cordarone had a 20% decrease in clearance. Prior surgery both increased the sensitivity to fluindione, reflected by a 50% reduction in IC 50, and decreased the sigmoidicity coefficient γ by about 50% so that the increase in the concentration-response curve is more gradual in these patients. The influence of deroxat on γ was the opposite, with a steeper curve for these patients indicating an on/off type of response. We performed a small stability study to assess the ability of the sparse design to estimate the PK parameters, and found that CL and V could be correctly estimated (see Supplementary Material), consistent with the low correlations reported between the estimates of the PK parameters (−0.16 for the correlation between the estimates of V and CL). The correlations were higher between the PD parameters of the Imax model (corr(IC50,Imax)=0.6 and corr(IC50, γ)= −0.73), suggesting that the design may not be as informative for the PD. All other correlations were lower than 0.35. The robustness of the estimates was checked by changing seeds and initial conditions. Shrinkage was large for most parameters, reflecting the relative lack of information in this sparse design: V (44%), CL (32%), IC50 (47%), kout (63%), I0 (86%) and γ (80%). The ε-shrinkage was 31% for PK and 39% for PD. Diagnostic plots are shown in figures 2 and 3. Graphs of the npde (normalised prediction distribution errors) versus time and dose, which are more appropriate than VPC because of the heterogeneous design [22, 23], are shown in Figure 3; prediction bands have been overlayed to indicate model predictions. The two upper graphs show the npde versus time, for fluindione (left) and INR (right), and indicate good model adequacy for fluindione, while for INR the model slightly underpredicts the last time-point. The two lower graphs show the npde versus model predictions; the model can be seen to perform adequately on average both for PK and PD, while variability is sometimes under or overestimated. Individual graphs are shown for 12 subjects from different clinical departments (Figure 4: fluindione concentrations; Figure 5: INR). The dotted line in the individual plots for INR show the target therapeutic range, while vertical bars are drawn to show the doses received (the scale for doses is on the right-hand axis). For most subjects, the model is able to reproduce both PK and PD measurements adequately, describing even complex profiles. In a few cases, (eg subject 1086, topmost panel, right), the PK is very well predicted but the PD shows unexplained fluctuations, with INR starting to decrease despite stable doses and concentrations. Using the steady-state approximation with the population parameters, we found that doses of 7.5 and 10 mg ensure an INR within the therapeutic range for a typical patient (weight 65 kg, age 85 kg), regardless of gender. IIV however is large, and often at least 2 doses provide an INR within the range. Table 3 shows dose recommendations depending on individual covariates, obtained by simulations under the model. In each setting, when taking into account IIV, this average dose is valid in about 20% of the simulations, while a dose within +/−2.5 mg of this dose is recommended in about 50% of the simulations. |
Fluindione is an AVK used mostly in France, where it is regarded as an interesting alternative to the warfarin; contrary to warfarin, fluindione is not a racemic mixture and its longer half-life is considered to help stabilise INR levels [24]. As other anticoagulant drugs however, it is a difficult drug to adjust. In a previous study called ADAP, we investigated the pharmacokinetics and pharmacodynamics of fluindione in a general population of patients initiating treatment [25, 26]. In these younger patients (mean 60 years, range 29–89), we demonstrated not only large IIV, but also, through a follow-up study recording dose changes and evolution of anticoagulation after discharge from the hospital, a sizeable intraindividual variability. This variability led to fluctuations in the anticoagulation level even in patients thought to be stabilised when leaving the hospital [27]. In the present study, we used a PK/PD model closely related to the one developed in ADAP. The main differences are the absorption model, which was previously assumed to a bolus dose and which we fixed here, and the use of a Hill model to represent drug effect. The estimates of CL and V were very close to the value estimated assuming an IV bolus, but the statistical criterion was slightly better with an oral absorption phase. The estimates of the PK parameters however are quite different from previously [25, 26]. In the final model, V was estimated to be around 8 L, while CL was 0.1 L.hr−1. In the ADAP study on the other hand, we estimated these parameters to be respectively 37 L and 0.49 L.hr−1. Both sets of parameters however give the same estimate for half-life, 56 h versus 52 h previously. The increased exposure was also apparent in the measured concentrations: in the ADAP study, subjects received daily doses of fluindione, with starting doses of 15 to 20 mg, and concentrations after 5 days of treatment were around 0.8 to 1.2 mg.L−1, while in PREPA concentrations at day 5 range from 1 to over 8 mg.L−1 as shown in figures 1 and 4. In addition, starting doses were lower, most patients receiving 10 mg initially or less. Thus, compared to the previous study, we observe a marked increase in the exposure to fluindione. Since fluindione is administered orally, the reported V and CL are apparent parameters, so an explanation to this discrepancy with previous results is a difference in bioavailability between the two studies. However, this would require a more than four-fold increase in bioavailability in elderly patients compared to the younger population previously studied. Fluindione is to some extent cleared by the liver, with the hepatic metabolism of fluindione appearing to be mediated by CYP2C9 [28], but is mainly renally excreted, so that we do not expect first-pass effect to be a major determinant of drug concentrations; the increase in drug exposure would then be driven by a dramatic increase in the absorption process. Changes in plasma protein binding could be another possibility. For all drugs eliminated primarily by the liver total exposure is independent of protein binding but, like fluindione, oral drugs eliminated by nonhepatic high extraction ratio routes exhibit changes in unbound drug exposure when protein binding changes. This would not be expected to affect exposure to such an extent since fluindione does not exhibit a particularly high extraction ratio [29]. The smaller volume of distribution might be the consequence of the combination of a lowered volume of tissue with an increase of the fraction unbound in tissue (V=Vp
+ Vt fu
/fut
), both clinically relevant in an elderly population with a lipophilic drug [30]. Modification, either increase or decrease, of transit times in this heavily medicated population could also explain an increase in the fraction absorbed by changing the dissolution of fluindione; indeed, 117 patients (89%) received drugs modifying transit, and slower intestinal transit times are frequently observed in the elderly. The alteration in PK exposure could therefore be due to a combination of factors [30] and warrants further exploration in a controlled study. Dose reduction with age is also observed for other oral anticoagulants [18, 24]. The latter study, although admittedly retrospective, included over 22 000 patients, and found that patients aged 80 years or older required doses one-third to one-half of those given to patients younger than 50. For fluindione, Mahé et al. observed in a retrospective study that patients over 75 years old required a lower dose of fluindione than younger patients for a comparable INR [31], which can be interpreted as due to modifications in exposure in the light of our results. For the PD parameters, we found an estimate of IC50 about 60% higher than in the ADAP study (2.18 mg.L−1 instead of 1.35 mg.L−1), but still within the same range, suggesting that elderly patients have a similar sensitivity to fluindione despite having an increased exposure. The present study provides a rationale explaining the findings of others that lower doses are required in elderly patients, by linking them to PK, and underscores the importance of having both PK and PD measurements in order to separate pharmacokinetic and pharmacodynamic changes. kout on the other hand was noticeably smaller (0.03 versus 0.18 hr−1), indicating a slower turnover than in younger patients. We used the model and parameter estimates to predict a dose that should be given in order to maintain an INR within the therapeutic range at steady-state. Consistent with the observation that the concentrations were higher in this population of elderly patients than in the population we previously studied [25, 26], the model predicted relatively low doses, suggesting a daily 10 mg dose, or an alternance of 5 and 10 mg doses, should be a safe starting dose for most patients. This is also in line with the maintenance doses observed in the retrospective study by Mahé [31]. Fluindione however is still packaged as 20 mg pills, which are in practice difficult to divide for routine treatment. The present study was targeted towards elderly patients during hospitalisation, since this population is both more likely to receive anticoagulant drugs and more fragile, being often polymedicated and with comorbidities. These patients are therefore more susceptible to bleeding and dose adjustment for oral anticoagulants is particularly difficult [32]. Given its observational nature and the short duration, the PREPA study was not specifically designed to investigate risk factors for clotting or bleeding; a more detailed analysis of the 8 bleeding events which occurred in the subset of patients with atrial fibrillation can be found in [20]. A major objective of the present study was to identify covariates that could explain some of the variability in the response. We found the pharmacokinetic parameters to be influenced by gender, weight and age, as well as prior administration of cordarone (amiodarone) while the only covariates influencing pharmacodynamic parameters were prior surgery and administration of deroxat. We did not find any relationship with time-varying comedications, but this may be due to the heterogeneity of the population and to the small sample size. Cordarone has been reported to increase the haemorragic risk [33], which could relate to the 20% reduction we find, translating into an increased exposure. We did not find a relationship with anti-infective agents (antibiotics and anti-fungal drugs) as in that study; nearly half of the patients received an antibiotic at some point during treatment, but it is possible that the analysis could not pick up a relationship especially if the influence is delayed, since we considered only an on/off type of relationship. Also the sample size was too small to consider each drug separately so we pooled the different antibiotics and the different dosages for the analyses. About a third of the patients took part in the optional pharmacogenetic study. We found no relationship between the 3 genetic polymorphisms and PK or PD parameters. The influence of CYP2C9 on PK through metabolism has been described for warfarin [34]; for fluindione, a recent study in healthy volunteers showed a lower clearance in carriers of the *3 allele [28], while the influence on the response is expected to be related to VKORC1 [35, 28]. We were not able to confirm these relationships here, perhaps due to the small number of subjects. Most patients did not remain in the study long enough to reach a stable INR. However, even within the duration of their stay, we noted that dose changes occurred too often and sometimes irrationally. In particular, the long time-course of INR evolution after AVK did not appear well anticipated by the prescribing clinicians. This is apparently partly due to a lack of follow-up, as doses are frequently modified based only on the latest INR, without considering the pattern of doses given and INRs measured since the beginning of the treatment. In several countries specialised anticoagulation clinics have been set up to help manage anti-vitamin K drugs and have been shown to improve management of anticoagulation treatment [8]; a pilot clinic now exists in France [36]. A perspective of the present work could be to develop a software helping clinicians to anticipate the future evolution of anticoagulation, by producing plots based on individual INR measurements and dosing regimen [37, 38]. In conclusion, the PREPA observational study highlighted that dose adjustement for AVK agents is still a major issue, especially in the elderly. The complexity of INR dynamics and the resulting delay between the time-course of the drug and the clinical variations of anticoagulation levels make it difficult to anticipate changes in INR, so that adjustements in doses must be made after taking into account the evolution over several days and not a single measurement. Elderly patients should be treated as a special population presenting a noticeably increased pharmacokinetic exposure to the drug. They should receive significantly lower doses and the onset of treatment should remain conservative. |
4.1. Data The PREPA study was a prospective, observational multicenter study conducted between September 2005 and September 2007, recruiting consecutive patients hospitalised in 6 medical and 1 surgical (cardiac) acute-care units from 3 French hospitals. Patients, 80 or older, were prescribed fluindione after at least 2 weeks off oral anticoagulants. Exclusion criteria were: contraindication to fluindione treatment due to hypersensitivity to indanedione derived drugs, incompatible comedication, inclusion in another therapeutic trial, expected length of stay in hospital less than 3 days. The study was approved by the Ethics committee from Hospital Européen Georges Pompidou (HEGP), and all participants provided written informed consent in accordance with the Declaration of Helsinki. The clinical trial has been registered on the public registry ClinicalTrials.gov. Patients were followed by clinicians according to the local clinical practices. In particular, doses were adjusted based on routine measurements of INR, without using any dosing algorithm. Fluindione was administered in the evening, usually around 6 p.m. Blood samples for the measurement of INR were taken in the morning, according to the usual practice in the participating centers for therapeutic monitoring: before the first administration of fluindione (day 0), and after 2, 4, 6 and 8 doses of fluindione; additional samples were obtained twice a week after that for therapeutic monitoring. The date of each sample was recorded by the nurse, and the time of the sample when it was outside of the window 9–11 a.m. Each time a blood sample was taken for the measurement of INR and coagulation factors, an additional 5 mL blood vial was drawn for measuring fluindione concentrations, except at day 0, where fluindione concentrations were measured only in patients who had received a dose of an oral anticoagulant within the two weeks immediately preceding the first dose of fluindione in the study. As an ancillary optional study, which required a separate informed consent, an additional blood sample was taken for genotyping. Genetic polymorphisms for two CYP2C9, which are known to influence the pharmacokinetics of warfarin [34] and acenocoumarol [35], as well as a genetic polymorphism in the gene coding for vitamin K epoxide reductase complex subunit 1 (VKORC1) [39], the target of AVK treatment and involved in the response [40], were determined. Analytical details are available in the Supplementary material. For each patient, the following variables were recorded at the inclusion visit by a clinician involved in the study: age, gender, weight, weight changes within the 6 months preceding the inclusion visit, ADL, Charlson comorbidity score, MMS, reason for fluindione treatment, therapeutic range for INR, major comorbidities, cardiac surgery. A number of biological variables were also measured at baseline: albumin, C-reactive protein (CRP), haematocrit levels, protidaemia, natremia. Creatinine clearance (CRCL) was computed from age (year) and creatinine clearance using the following formula [41]:
Renal function was classified as normal (CLCR≥60 ml/mn), moderately impaired (CLCR between 30–60 ml/mn) and severely impaired (CLCR<30 ml/mn). Patients were defined as suffering from malnutrition when they had recent severe weight loss (over 10% of weight), a body mass index lower than 18, or albumin levels lower than 30. Finally, high levels of CRP (CRP>50 mg.L−1) were considered to be a sign of current hypercatabolism. One important objective in this group of elderly inpatients was to describe and take into account the many comedications these patients received. Comedications given to each patient were recorded at the initial visit, and changes were documented throughout the study. Comedications received were classified according to their influence on INR, using the classification proposed by Holbrook [42] (given in Supplementary Table S2). Drugs were first classified according to whether they increase the thrombotic risk or the haemorragic risk; the second group was divided further into drugs increasing the INR (class B2) versus drugs acting independently (class B1). Nonsteroidal anti-inflammatory drugs (group B12) and diuretics were also considered for their potential effect on pharmacokinetic parameters. For each class and each subject, we defined a daily indicator variable. Cordarone, amiodarone and deroxat, because of their long half-life, were considered separately and assumed to remain active for 1 month (cordarone and amiodarone) and 15 days (deroxat). Only therapeutic classes which were received by at least 10 and at most 122 patients were considered in the analysis. 4.2. Model building Non-linear mixed effect models were used in this analysis; the jth observation in subject i, y
ij, is modelled as:
where ψi
denotes the individual parameters, f the structural model and g the residual error model ( εij
~
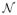 (0, 1)). We assume that ψi
depends on individual covariates ci
, a vector of fixed effects μ and a vector of individual random effects ηi
through a function h:
The parameters were estimated through the Stochastic Approximation EM algorithm (SAEM) implemented in the MONOLIX software [43] (version 3.2, release 1), running on a Linux PC (Kubuntu 10.10). All other statistical analyses were performed in R (2.11) [44]. Base model selection We first studied the PK of fluindione alone. The residual variability was modelled using a combined error model. The structural model was built by selecting the best model amongst one and two-compartmental models with first or zero-order absorption, with or without lag-time. The evolution of INR was modelled using a turnover model, describing the evolution of coagulation factor activity F (t) = 1/INR(t) through the following equation:
The model was parameterised in terms of k
out and INR0 = 1/F
0 where, in the absence of drug, we have the relationship: F
0
k
out = R
syn. Imax was assumed to follow a logit distribution while all the other parameters had a log-normal distribution. For each parameter we tested whether IIV could be removed from the model, and we also tested correlations between parameters by introducing covariances. To select structural and variability models, we used appropriate likelihood ratio tests (LRT), based on the log-likelihood computed using importance sampling. Covariate model building We examined the relationships between parameters and covariates, first in the PK model alone, then in the PK/PD model. Covariate model building was done by exploring the relationships between covariates and estimated individual parameters (obtained as the conditional modes of the individual distribution) through linear regression for the continuous covariates and parametric tests for the categorical covariates; all candidate covariates were included in a full model, using power functions for continuous covariates; the model was then pruned down by removing covariate effects for which the p-value of the Wald test was larger than 0.05, starting with the covariates which had the largest p-value. A non-significant Wald test indicates a high estimation error for the corresponding parameter. The same approach was used for the full PK/PD model with the best PK model, re-estimating all parameters. The final PK/PD model was evaluated using standard diagnostic graphs provided by MONOLIX. We also computed the shrinkage, as, for the kth- parameter:
where
 is the estimated kth random effect in subject i. The ε-shrinkage was computed separately for PK and PD:
where IWRES denotes the individual weighted residuals. In the presence of high shrinkage, diagnostic plots based on individual estimated parameters are less informative [ 45]. Covariates missing in more than 14 subjects (10%) were excluded from the analysis; the other missing covariates were imputed to the mean value for continuous variables and to a value randomly sampled for the discrete covariates. The final model was checked using multiple imputation [46], using the package mice for R [47]. An additional exploratory analysis investigated relationships between the PK/PD parameters and the genetic covariates in subsets of the data. Steady-state dose Fluindione is provided as 20 mg tablets, which can be cut in four 5 mg pieces; dosage is often alternated (eg 10 mg one day and 5 the next). An estimate of the steady-state dose for a given patient can be obtained through the following equations, where C ss denotes the average concentration that would be obtained for regular doses given every 24 hours, and INR ss denotes the steady-state INR that would be reached assuming the concentration remained equal to C ss:
We used the second equation to predict INRss given a set of PK/PD parameters, for doses ranging from 2.5 to 30 mg per day; a dose was considered to be adequate if INRss was within the desired therapeutic range of 2–3. We also defined a recommended dose by using 1000 Monte-Carlo simulations taking into account IIV; for each simulation, the recommended dose was the dose yielding the INRss closest to 2.5. An associated probability is the percentage of simulations recommending that dose. |
|
This study was supported by a grant from the Groupement d’Intérêt Scientifique (GIS) Longévité, and the genotyping analyses were financed by a grant from the Comité d’Orientation Stratégique et de Suivi des Essais Cliniques (COSSEC, INSERM, Paris, France). The promoter of the study was the Institut de la Santé et de la Recherche Médicale (INSERM, Paris, France).
Estelle Mottez (INSERM) was instrumental in taking care of the promotion of the study. The Centre d’Investigation Clinique (CIC) of the Bichat hospital (Paris, France) kindly ensured the monitoring of the study. We would like to thank in particular Dr Hayk Papayan and Valérie Vignali for their involment in the study follow-up. We would also like to thank the Centre de Ressources Biologiques (CRB Bichat) and especially Dr Joëlle Benessiano for taking care of the DNA samples. Finally, we would like to thank the nursing staff who ensured daily sample collection and the staff from the different analytical departments involved in the study.
|
Footnotes |
1.
Lip G,
Hart R,
Conway D,
Antithrombotic therapy for atrial fibrillation
Br Med J
325
1022
25
2002
. 2.
Camm A,
Kirchhof P.
Lip G,
Schotten U,
European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery
Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the european society of cardiology (ESC)
Eur Heart J
31
2369
429
2010
. 3.
Kearon C,
Kahn SR,
Agnelli G.
Goldhaber S,
Raskob GE,
Comerota AJ,
Antithrombotic therapy for venous thromboembolic disease. (American College of Chest Physicians evidence-based clinical practice guidelines, 8th edition)
Chest
133
454S
545S
2008
. 4.
Vahanian A.
et al.
Guidelines on the management of valvular heart disease: The task force on the management of valvular heart disease of the European Society of Cardiology
Eur Heart J
28
230
68
2007
. 5.
Hart R,
Benavente O.
Mc Bride R,
Pearce L,
Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis
Ann Intern Med
131
492
501
1999
. 6.
Lagerstedt C,
Olsson C,
Fagher B,
Oquist B,
Albrechtsson V,
Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis
Lancet
2
515
18
1985
. 7.
Holford N,
Clinical pharmacokinetics and pharmacodynamics of warfarin. understanding the dose–effect relationship
Clin Pharmacokinet
11
483
504
1986
. 8.
Ansell J,
Hollowell J,
Pengo V.
Martinez-Brotons F,
Caro J,
Drouet L,
Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: the international study of anticoagulation management (ISAM)
J Thromb Thrombolysis
23
83
91
2007
. 9.
Levine M,
Raskob G.
Landefeld S,
Kearon C,
Hemorrhagic complications of anticoagulant treatment
Chest
119
108s
21s
2001
. 10.
Pouyanne P,
Haramburu F.
Imbs J,
Bégaud B,
Admissions to hospital caused by drug reactions : cross sectional incidence study
Br Med J
320
1036
2000
. 11.
Agence Française de Sécurité Sanitaire des Produits de Santé. [good management practices for oral anticoagulant overdose, situations of hemorrhagic risk and hemorrhagic events in patients taking oral anticoagulants in the ambulatory and hospital setting]
J Mal Vascul
33
202
13
2008
. 12.
Linkins L,
Choi P,
Douketis J,
Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis
Ann Intern Med
139
893
900
2003
. 13.
Hutten B,
Lensing A.
Kraaijenhagen R,
Prins M,
Safety of treatment with anticoagulants in the elderly. A systematic review
Drugs Aging
14
303
12
1999
. 14.
Ansell J,
Weitz J,
Comerota A,
Advances in the therapy and the management of antithrombotic drugs for venous thromboembolism
Hematology
23
266
84
2000
. 15.
Fihn S,
Callahan C,
Martin D.
Mc Donell M,
Heniko J,
White R,
The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics
Ann Intern Med
124
970
9
1996
. 16.
Palareti G.
Cosmi B,
Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients
Thromb Haemost
102
268
78
2009
. 17.
Evans A,
Davis S,
Kilpatrick C.
Gerraty R,
Campbell D,
Greenberg P,
The morbidity related to atrial fibrillation at a tertiary centre in one year: 9.0% of all strkes are potentially preventable
J Clin Neurosci
9
268
72
2002
. 18.
Hylek E,
Oral anticoagulants. Pharmacological issues for the elderly
Clin Geriatr Med
17
1
13
2002
. 19.
Hylek E,
Regan S,
Go A.
Hughes R,
Singer D,
Skates S,
Clinical predictors of prolonged delay in return of the international normalized ratio to within the therapeutic ranged after excessive anticoagulation with warfarin
Ann Intern Med
135
393
400
2001
. 20.
Bonnet-Zamponi D.
et al.
Heparin bridging therapy and bleeding events in octogenarians inpatients with atrial fibrillation starting anticoagulation: Results of an ancillary study
J American Geriatr Soc
2011
in press
. 21.
Warot D.
et al.
Beraprost sodium-fluindione combination in healthy subjects: pharmacokinetic and pharmacodynamic aspects
Fundam Clin Pharmacol
14
231
6
2000
. 22.
Brendel K,
Comets E,
Laffont C,
Laveille C,
Mentré F,
Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide
Pharm Res
23
2036
49
2006
. 23.
Comets E,
Brendel K,
Mentré F,
Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R
Comput Methods Programs Biomed
90
154
66
2008
. 24.
Fihn S.
et al.
Comparison of control and stability of oral anticoagulant therapy using acenocoumarol versus phenprocoumon
Thromb Haemost
90
260
6
2003
. 25.
Mentré F.
et al.
Population pharmacokinetic–pharmacodynamic analysis of fluindione in patients
Clin Pharmacol Ther
63
64
78
1998
. 26.
Comets E.
et al.
Modeling INR data to predict maintenance fluindione dosage
Ther Drug Monit
20
631
639
1998
. 27.
Comets E.
et al.
Prediction of fluindione maintenance dosage hampered by large intraindividual variability
Ther Drug Monit
22
668
75
2000
. 28.
Verstuyft C.
et al.
A PK-PD model for predicting the impact of CYP2C9 and VKORC1 polymorphisms on fluindione and acenocoumarol during induction therapy
Clin Pharmacol Ther
2011
in press
. 29.
Benet L.
Hoener BA,
Changes in plasma protein binding have little clinical relevance
Clin Pharmacol Ther
71
115
21
2002
. 30.
Currie G,
Wheat J,
Kiat H,
Pharmacokinetic considerations for digoxin in older people
Open Cardiovasc Med J
5
130
5
2011
. 31.
Mahé I,
Grenard AS,
Joyeux N,
Caulin C,
Bergmann JF,
Management of oral anticoagulant in clinical practice: a retrospective study of 187 patients
J Gerontol A Biol Sci Med Sci
59
1339
42
2004
. 32.
Vogel T,
Coriol V,
Kaltenbach G,
Kiesmann M,
Berthel M,
Prospective study of oral anticoagulation control in 110 very elderly hospitalized patients and of risk factors for poor control] [Article in French
Presse Med
37
1723
30
2008
. 33.
Siguret V,
Esquirol C,
Debray M.
Gouin I,
Andreux JP,
Pautas E,
Excess antivitamin k in elderly hospitalised patients aged over 70. a one-year prospective survey] [Article in French
Presse Med
32
972
7
2003
. 34.
Aithal GP,
Day CP.
Kesteven PJL,
Daly AK,
Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications
Lancet
353
717
9
1999
. 35.
Verstuyft C.
et al.
Genetic and environmental risk factors for oral anticoagulant overdose
Eur J Clin Pharmacol
58
739
45
2003
. 36.
Lévesque H.
Borg JY,
Anticoagulant clinic: a tool for reduce bleeding complications of oral anticoagulant treatment] [Article in French
Rev Med Intern
24
75
7
2003
. 37.
Manotti C,
Moia Palareti G,
Pengo V,
Ria L,
Dettori A,
Effect of computer-aided management on the quality of treatment in anticoagulated patients: a prospective, randomized, multicenter trial of APROAT (Automated Program for Oral Anticoagulant Treatment)
Haematologica
86
1060
70
2001
. 38.
Saint-Marcoux F,
Vandierdonck S,
Prémaud A.
Debord J,
Rousseau A,
Marquet P,
Large scale analysis of routine dose adjustments of mycophenolate mofetil based on global exposure in renal transplant patients
Ther Drug Monit
33
285
94
2011
. 39.
Rost S.
et al.
Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2
Nature
427
537
41
2004
. 40.
Bodin L.
et al.
Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity
Blood
106
135
40
2005
. 41.
Levey A.
et al.
A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation
Ann Intern Med
16
461
70
1999
. 42.
Holbrook AM.
et al.
Systematic overview of warfarin and its drug and food interactions
Arch Intern Med
165
1095
106
2005
. 44.
R Development Core Team
R: A language and environment for statistical computing
R Foundation for Statistical Computing
Vienna, Austria
2004
URL http://www.R-project.org
. 45.
Karlsson M.
Savic R,
Diagnosing model diagnostics
Clin Pharmacol Ther
82
17
20
2007
. 46.
Little R.
Rubin D,
Statistical analysis with missing data
John Wiley & Sons, Inc
Hoboken, New Jersey
2002
2
. 47.
van Buuren S.
Groothuis-Oudshoorn K,
MICE: Multivariate imputation by chained equations in R
J Stat Soft
2010
in press
. 48.
Aymard G,
Legrand M,
Comets E,
Mentré F,
Diquet B,
Rapid and simple micromethod for the quantification of fluindione in human plasma using high–performance liquid chromatography
J Chromatogr B
707
169
173
1998
. 49.
Rieder MJ.
et al.
Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose
N Engl J Med
352
2285
93
2005
. 50.
Lacut K.
et al.
Vitamin K epoxide reductase genetic polymorphism is associated with venous thromboembolism: results from the EDITH study
J Thromb Haemost
5
2020
4
2007
. |
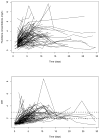 | Figure 1 PKPD data collected in the 131 patients from the PREPA study: (top) concentration of fluindione versus time in the study (in days); (bottom) INR versus time in the study. Dotted lines delineate the therapeutic range for the patients in PREPA (2–3). |
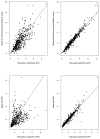 | Figure 2Goodness-of-fit plots: (top) observed versus predicted fluindione concentrations; (bottom) observed versus predicted INR. The plots on the left were obtained with population parameter estimates, while the plots on the right were obtained with individual (more ...) |
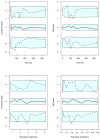 | Figure 3Goodness-of-fit plots: npde with prediction intervals, for fluindione (left) and INR (right) versus time (top) and predictions (bottom). The blue areas correspond to the prediction intervals for the median (central band) and for the limits of the 95% (more ...) |
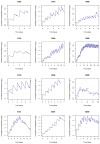 | Figure 4 Individual fits for 12 subjects, fluindione concentrations. Patient numbers starting with 1 indicate patients recruited in the cardiology department, with 3 or 4 one of the two geriatric departements, and with 5 the internal medicine department. |
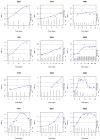 | Figure 5 Individual fits for the same subjects, INR, superimposed with the daily dosing pattern as vertical bars (scale for doses are on the right-hand axis). |
 | Table 1 Demographic and biologic variables in the 131 patients from the PREPA study. |
 | Table 2 Parameter estimates for the PK/PD model without covariate (base model) and for the final model. Interindividual variability is given as coefficient of variation (CV), and the relative estimation error (RSE) is also shown. |
 | Table 3Recommended dose depending on different patient profiles, defined as the dose yielding a steady-state value closest to 2.5. The last column represents the percentage of time that the corresponding dose is selected over the 1000 Monte-Carlo simulations (more ...) |
|




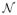 (0, 1)). We assume that ψi
depends on individual covariates ci
, a vector of fixed effects μ and a vector of individual random effects ηi
through a function h:
(0, 1)). We assume that ψi
depends on individual covariates ci
, a vector of fixed effects μ and a vector of individual random effects ηi
through a function h:



 is the estimated kth random effect in subject i. The ε-shrinkage was computed separately for PK and PD:
is the estimated kth random effect in subject i. The ε-shrinkage was computed separately for PK and PD:

