Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis.
Résumé
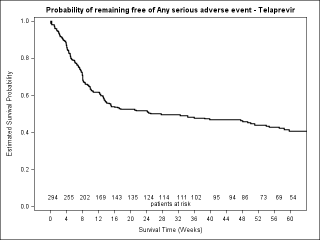
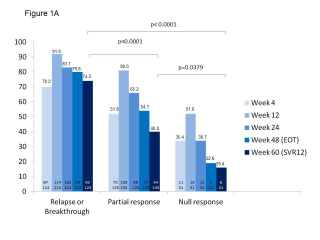
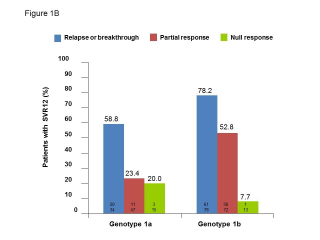
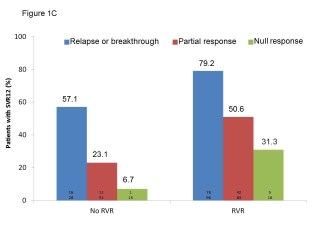
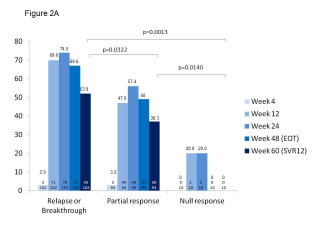
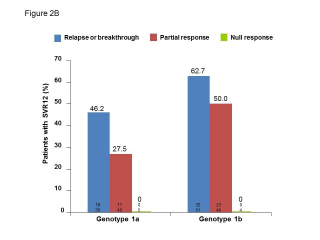
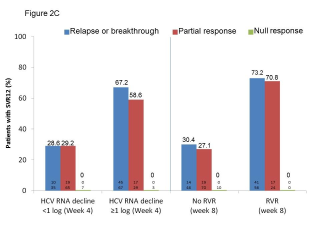
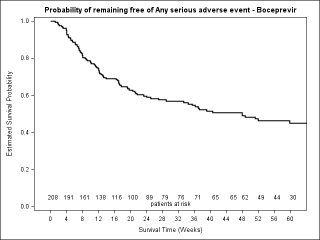
BACKGROUND & AIMS: We investigated the effectiveness of the protease inhibitors peginterferon and ribavirin in treatment-experienced patients with hepatitis C virus (HCV) genotype 1 infection and cirrhosis. METHODS: In the Compassionate Use of Protease Inhibitors in Viral C Cirrhosis study, 511 patients with HCV genotype 1 infection and compensated cirrhosis who did not respond to a prior course of peginterferon and ribavirin (44.3% relapsers or patients with viral breakthrough, 44.8% partial responders, and 8.0% null responders) were given either telaprevir (n = 299) or boceprevir (n = 212) for 48 weeks. We assessed percentages of patients with sustained viral responses 12 weeks after therapy and safety. This observational study did not allow for direct comparison of the 2 regimens. RESULTS: Among patients given telaprevir, 74.2% of relapsers, 40.0% of partial responders, and 19.4% of null responders achieved SVR12. Among those given boceprevir, 53.9% of relapsers, 38.3% of partial responders, and none of the null responders achieved SVR12. In multivariate analysis, factors associated with SVR12 included prior response to treatment response, no lead-in phase, HCV subtype 1b (vs 1a), and baseline platelet count greater than 100,000/mm(3). Severe adverse events occurred in 49.9% of cases, including liver decompensation, severe infections in 10.4%, and death in 2.2%. In multivariate analysis, baseline serum albumin level less than 35 g/L and baseline platelet counts of 100,000/mm(3) or less predicted severe side effects or death. CONCLUSIONS: Relatively high percentages of real-life, treatment-experienced patients with HCV genotype 1 infection and cirrhosis respond to the combination of peginterferon and ribavirin with telaprevir or boceprevir. However, side effects are frequent and often severe. Baseline levels of albumin and platelet counts can be used to guide treatment decisions. ClinicalTrials.gov number: NCT01514890.
Fichier principal
 GASTRO-D-13-02129_article_revised.pdf (287.31 Ko)
Télécharger le fichier
GASTRO-D-13-02129_article_revised.pdf (287.31 Ko)
Télécharger le fichier
 GASTRO-D-13-02129_Figure_1_supplementary.JPG (66.75 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_1_supplementary.JPG (66.75 Ko)
Télécharger le fichier
 GASTRO-D-13-02129_Figure_1A.JPG (116.03 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_1A.JPG (116.03 Ko)
Télécharger le fichier
 GASTRO-D-13-02129_Figure_1B.JPG (75.75 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_1B.JPG (75.75 Ko)
Télécharger le fichier
 GASTRO-D-13-02129_Figure_1C.JPG (76.41 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_1C.JPG (76.41 Ko)
Télécharger le fichier
 GASTRO-D-13-02129_Figure_2A.JPG (110.34 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_2A.JPG (110.34 Ko)
Télécharger le fichier
 GASTRO-D-13-02129_Figure_2B.JPG (71.41 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_2B.JPG (71.41 Ko)
Télécharger le fichier
 GASTRO-D-13-02129_Figure_2C.JPG (106.97 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_2C.JPG (106.97 Ko)
Télécharger le fichier
 GASTRO-D-13-02129_Figure_2_supplementary.JPG (65.63 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_2_supplementary.JPG (65.63 Ko)
Télécharger le fichier
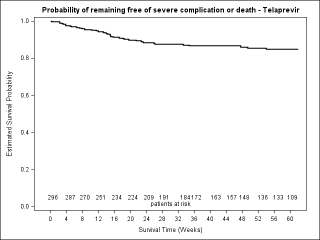 GASTRO-D-13-02129_Figure_3_supplementary.JPG (65.63 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_3_supplementary.JPG (65.63 Ko)
Télécharger le fichier
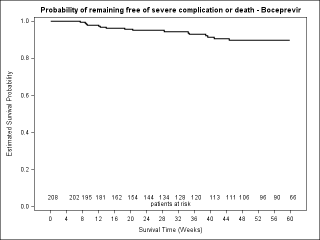 GASTRO-D-13-02129_Figure_4_supplementary.JPG (64.09 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Supplementary_material_revised.pdf (101.56 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Figure_4_supplementary.JPG (64.09 Ko)
Télécharger le fichier
GASTRO-D-13-02129_Supplementary_material_revised.pdf (101.56 Ko)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Format : Figure, Image
Origine : Fichiers produits par l'(les) auteur(s)
Loading...


