β-Bulges: extensive structural analyses of β-sheets irregularities.
Résumé
β-Sheets are quite frequent in protein structures and are stabilized by regular main-chain hydrogen bond patterns. Irregularities in β-sheets, named β-bulges, are distorted regions between two consecutive hydrogen bonds. They disrupt the classical alternation of side chain direction and can alter the directionality of β-strands. They are implicated in protein-protein interactions and are introduced to avoid β-strand aggregation. Five different types of β-bulges are defined. Previous studies on β-bulges were performed on a limited number of protein structures or one specific family. These studies evoked a potential conservation during evolution. In this work, we analyze the β-bulge distribution and conservation in terms of local backbone conformations and amino acid composition. Our dataset consists of 66 times more β-bulges than the last systematic study (Chan et al. Protein Science 1993, 2:1574-1590). Novel amino acid preferences are underlined and local structure conformations are highlighted by the use of a structural alphabet. We observed that β-bulges are preferably localized at the N- and C-termini of β-strands, but contrary to the earlier studies, no significant conservation of β-bulges was observed among structural homologues. Displacement of β-bulges along the sequence was also investigated by Molecular Dynamics simulations.
Fichier principal
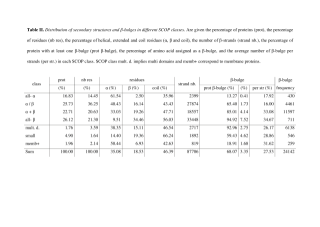 Table_II.pdf (112.13 Ko)
Télécharger le fichier
Craveur_Joseph_Rebehmed_de_Brevern_ProtSci_2013_preprint.pdf (755.63 Ko)
Télécharger le fichier
Supplementary_Material.doc (523.5 Ko)
Télécharger le fichier
Table_I.pdf (453.94 Ko)
Télécharger le fichier
Table_III.pdf (102.97 Ko)
Télécharger le fichier
Table_IV.pdf (158.5 Ko)
Télécharger le fichier
Table_V.pdf (102.78 Ko)
Télécharger le fichier
Table_VI.pdf (68.06 Ko)
Télécharger le fichier
Table_II.pdf (112.13 Ko)
Télécharger le fichier
Craveur_Joseph_Rebehmed_de_Brevern_ProtSci_2013_preprint.pdf (755.63 Ko)
Télécharger le fichier
Supplementary_Material.doc (523.5 Ko)
Télécharger le fichier
Table_I.pdf (453.94 Ko)
Télécharger le fichier
Table_III.pdf (102.97 Ko)
Télécharger le fichier
Table_IV.pdf (158.5 Ko)
Télécharger le fichier
Table_V.pdf (102.78 Ko)
Télécharger le fichier
Table_VI.pdf (68.06 Ko)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Format : Autre
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)