Ciclosporin population pharmacokinetics and Bayesian estimation in thoracic transplant recipients
Résumé
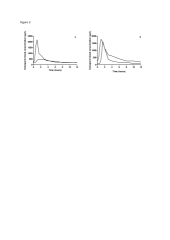
Background and objectives: Therapeutic drug monitoring of ciclosporin has been recognized as an essential tool in the management of allograft transplant recipients, as it could help improve their outcome. However, there is still no consensus about the optimal method for monitoring ciclosporin after thoracic transplantation. Better knowledge of the pharmacokinetics of ciclosporin in thoracic transplant patients and design of tools dedicated to ciclosporin monitoring could help its practice and its outcome in this population of patients. The aims of this study were: (i) to investigate the population pharmacokinetics of ciclosporin in thoracic (heart or lung) transplant patients, and study the influence of a range of potential covariates, including demographic, clinical and genetic factors, on pharmacokinetic parameters; and (ii) to develop a Bayesian estimator able to predict the individual pharmacokinetic parameters and exposures indices in this population of patients. Methods: The analysis was performed with 187 full pharmacokinetic profiles obtained in 57 lung and 19 heart transplant patients within the first year post-transplantation. A population pharmacokinetic model was developed by nonlinear mixed effect modeling using NONMEM (version 7.1) from an index dataset (118 profiles). On the basis of this population model and a limited number of blood samples, a Bayesian estimator able to determine ciclosporin area under the blood concentration-time curve during a dosage interval was built and evaluated in the validation dataset (69 profiles). Results: Ciclosporin pharmacokinetics was described using a two-compartment model with time-lagged first order absorption and first order elimination. The final population model included sex as a covariate: ciclosporin apparent oral clearance was on average 37% faster in male than in female patients (34.8 vs 25.4 L/h, p <0.001). Good predictive performance of the Bayesian estimator was obtained using three blood concentrations measured at 40 minutes, 2 and 4 hours post-dose, with a non-significant bias of -5% between the estimated and the reference trapezoidal area under the curve and a good precision (relative mean square error=13%). Conclusion: Ciclosporin population pharmacokinetic analysis in thoracic transplant patients (including patients with cystic fibrosis) showed a significant influence of sex on apparent clearance. The Bayesian estimator developed in this study yielded accurate prediction of ciclosporin exposure in this population throughout the first year post-transplantation. This tool may allow routine ciclosporin dose individualization.
Domaines
Sciences pharmaceutiques
Fichier principal
 Figure_3.pdf (82.41 Ko)
Télécharger le fichier
Art_A._PrA_maud.pdf (732.69 Ko)
Télécharger le fichier
Figure_1.pdf (362.19 Ko)
Télécharger le fichier
Figure_2.pdf (263.31 Ko)
Télécharger le fichier
Figure_3.pdf (82.41 Ko)
Télécharger le fichier
Art_A._PrA_maud.pdf (732.69 Ko)
Télécharger le fichier
Figure_1.pdf (362.19 Ko)
Télécharger le fichier
Figure_2.pdf (263.31 Ko)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)