Processing of human cathepsin D is independent of its catalytic function and auto-activation: involvement of cathepsins L and B.
Résumé
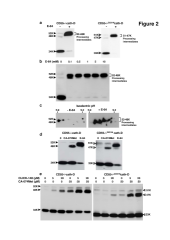
The current mechanism proposed for the processing and activation of the 52 kDa lysosomal aspartic protease cathepsin D (cath-D) is a combination of partial auto-activation generating a 51 kDa pseudo-cath-D, followed by enzyme-assisted maturation involving cysteine and/or aspartic proteases and yielding successively a 48 kDa intermediate and then 34 + 14 kDa cath-D mature species. Here we have investigated the in vivo processing of human cath-D in a cath-D-deficient fibroblast cell line in order to determine whether its maturation occurs through already active cath-D and/or other proteases. We demonstrate that cellular cath-D is processed in a manner independent of its catalytic function and that auto-activation is not a required step. Moreover, the cysteine protease inhibitor E-64 partially blocks processing, leading to accumulation of 52-48 kDa cath-D intermediates. Furthermore, two inhibitors, CLICK148 and CA-074Met, specific for the lysosomal cath-L and cath-B cysteine proteases induce accumulation of 48 kDa intermediate cath-D. Finally, maturation of endocytosed pro-cath-D is also independent of its catalytic function and requires cysteine proteases. We therefore conclude that the mechanism of cath-D maturation involves a fully-assisted processing similar to that of pro-renin.
Fichier principal
 Copie_de_Fig2fmaturation_et_E64.pdf (212.09 Ko)
Télécharger le fichier
Copie_de_Fig1_maturation.pdf (175.72 Ko)
Télécharger le fichier
Copie_de_Fig3_maturation_3.pdf (15.97 Ko)
Télécharger le fichier
Copie_de_Fig4endocytosis_2_cathDAsn231.pdf (177.69 Ko)
Télécharger le fichier
Copie_de_paper_JB_final.pdf (71.22 Ko)
Télécharger le fichier
Copie_de_Fig2fmaturation_et_E64.pdf (212.09 Ko)
Télécharger le fichier
Copie_de_Fig1_maturation.pdf (175.72 Ko)
Télécharger le fichier
Copie_de_Fig3_maturation_3.pdf (15.97 Ko)
Télécharger le fichier
Copie_de_Fig4endocytosis_2_cathDAsn231.pdf (177.69 Ko)
Télécharger le fichier
Copie_de_paper_JB_final.pdf (71.22 Ko)
Télécharger le fichier