| |
| J Neurochem. 2004 April; 89(2): 454–63. doi: 10.1111/j.1471-4159.2004.02356.x.Anti-PrP antibodies block PrPSc replication in prion-infected cell cultures by accelerating PrPC degradation Véronique Perrier,1* Jérôme Solassol,1 Carole Crozet,1 Yveline Frobert,2 Chantal Mourton-Gilles,3 Jacques Grassi,2 and Sylvain Lehmann1 1Institut de génétique humaine
CNRS : UPR1142, 141 Rue de la Cardonille
34396 MONTPELLIER CEDEX 5,FR 2Service de Pharmacologie et d'Immunologie
CEA Saclay, 91191 Gif sur Yvette,FR 3Institut de Biotechnologie-Pharmacologie
CNRS : UMR5094, BioRad, Université Montpellier I, Université Montpellier II - Sciences et Techniques du Languedoc, Faculté de pharmacie, 15 avenue Charles Flahault BP14491, 34093 Montpellier Cedex 05,FR |
The usage of anti-PrP antibodies represent one of the most promising strategy for the treatment of prion diseases. In the present study, we screened various anti-PrP antibodies, with the aim to identify those that will block PrPSc replication in prion infected cell culture. Two antibodies, SAF34 recognizing the flexible octarepeats region on HuPrP protein and SAF61 directed against PrP amino acid residues (144–152), not only inhibited PrPSc formation in prion-infected neuroblastoma cells but also decreased the PrPC levels in non infected N2a cells. In addition, treatment with both SAF34 and SAF61 antibodies decreased the PrPC and the PrPSc levels in the cells, synergistically. In presence of both antibodies, our results showed that the mode of action which leads to the disappearance of the PrPSc in cells is directly coupled to PrPC degradation by reducing the half-life of the PrPC protein. MeSH keywords: Animals, Antibodies, Monoclonal, pharmacology, Cell Line, Tumor, Dose-Response Relationship, Drug, Drug Evaluation, Preclinical, Drug Synergism, Humans, Mice, Neuroblastoma, drug therapy, metabolism, PrPC Proteins, immunology, metabolism, PrPSc Proteins, antagonists & inhibitors, immunology, Prion Diseases, immunology, therapy Author keywords: antibodies mechanism and therapeutic, anti-prion agent, prion diseases |
The prion agent lies at the heart of several fatal neurodegenerative diseases including Creutzfeldt-Jakob disease (CJD) in humans and Transmissible Spongiform Encephalopathies (TSE) in animals (Prusiner et al. 1998). The understanding of the molecular basis for prion diseases, such as the replication cycle of the infectious agent still remains unclear (Telling et al. 1996). A common feature in prion diseases is the accumulation of an abnormal isoform (PrPSc) of a host-encoded prion protein (PrPC) in the central nervous system. The differences in structure between the two isoforms, PrPC and PrPSc, are well characterized. Monomers as well as dimers of the recombinant PrPC exhibited a structure rich in α-helices (Riek et al. 1996; Donne et al. 1997; Knaus et al. 2001). By contrast, electron microscopy analysis of 2D crystals of purified hamster PrPSc confirmed the increasing content in β-sheets present in the abnormal isoform and revealed a structure organized in parallel β-helices (Caughey et al. 1991; Pan et al. 1993; Wille et al. 2002). Differences in biochemical properties between these two isoforms allows for their discrimination. For instance, PrPSc is resistant to partial digestion by proteinase-K whereas PrPC is completely hydrolyzed (Meyer et al. 1986). Concerns about prion disorders have been heightened by the appearance of the bovine spongiform encephalopathy (BSE) in Great Britain and in many herds from other european countries. In addition, 137 cases of young adults have developed new variant Creutzfeldt-Jakob disease (vCJD) after exposure to bovine prions, most likely through consumption of contaminated-beef product (Will et al. 1996). Although the total number of new vCJD cases seems to not follow the initial prediction by Ghani et al. (1998), we can not exclude an augmentation of cases in the future (Ghani et al. 1998). Currently, no effective therapy exists and the development of novel therapeutic strategies against prion diseases has become a priority. New chemical molecules discovered serendipitously like branched polyamines (Supattapone et al. 1999), phtalocyanines, porphyrins (Caughey et al. 1998), quinacrine and chlorpromazine (Korth et al. 2001) demonstrated their ability to cure the scrapie-infected neuroblastoma cells (ScN2a) from their PrPSc molecules and have been shown to diminish the infectious titer in mice. Quinacrine and chlorpromazine, well known for the treatment of malaria and various psychoses, are currently administered to patients suffering from sporadic CJD or vCJD. It remains to be determined if the drug quinacrine will prove effective in treating prion diseases in humans as it is not efficient in mice (Collins et al. 2002; Barret et al. 2003). Porphyrins gave the most interesting results in vivo and increased the survival time up to 165 % in mice depending of the protocol of administration, which suggests that this drug might be used as a post-exposure prophylactic treatment (Priola et al. 2000). In parallel, rational drug design strategies, which are the basis of most modern drug discoveries, are difficult to set up for prion diseases (Perrier et al. 2000). Until recently, this was due to the absence of a well-defined tertiary and/or quaternary structure for both PrPSc and PrPC isoforms, as well as a lack of knowledge of the replication cycle of the prion agent. Only antibodies directed against the prion protein can abrogate these barriers and might, therefore, represent the most promising therapeutic strategy for the treatment of prion diseases (White et al. 2003). Anti-PrP antibodies bind their target with a high affinity and seem to inhibit the replication cycle of the prion agent by disrupting the interaction between PrPC and PrPSc molecules (Enari et al. 2001; Peretz et al. 2001). In addition, scrapie pathogenesis is prevented in transgenic mice expressing anti-PrP antibody fragments, sustaining the development of a vaccination strategy (Heppner et al. 2001). Alternatively, by stimulating the innate immunity of mice with small CpG deoxyoligonucleotides, Sethi et al., succeeded to delay prion disease symptoms (Sethi et al. 2002). In the present study, we identified two antibodies, SAF34 and SAF61, that not only inhibited PrPSc formation in prion-infected neuroblastoma cells (N2a58/22L) but also decreased the PrPC levels in normal N2a58 cells. For the first time, our results show that the mode of action which leads to the disappearance of the PrPSc in cells is directly coupled to PrPC degradation by reducing the half-life of the PrPC protein in the presence of both antibodies. |
Reagents and Antibodies Pefabloc and proteinase K were purchased from Roche. Modified Eagle’s medium with L glutamine (MEM), Phosphate-buffered saline (PBS), trypsin and geneticin were from Invitrogen Life Technologies, Inc. RPMI 1640 medium and 35S Trans Label reagent were provided by ICN Pharmaceutical. Fetal Calf serum (FCS) was from BioWhittaker. Secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). All other chemicals were from Sigma. Monoclonal antibodies SAF32, SAF34, SAF37, SAF60, SAF61, SAF69 and SAF70 were generated in the laboratory of Jacques Grassi ( Demart et al. 1999). Scrapie-associated fibrills from infected hamster brains were inoculated to knock-out Prnp0/0 mice, as immunogen by using conventional procedures. These monoclonal antibodies were proved to recognize continuous epitopes as seen by checking their reactivity with PrP synthetic peptides, using enzyme immunoassay measurements ( Demart et al. 1999) (see Table 1 for epitope mapping). Monoclonal antibodies 3B5 and 11C6 were produced against human recombinant PrP and were kindly provided by Dr. Krasemann ( Krasemann et al. 1996a; Krasemann et al. 1996b). mAb to Glyceraldehyde-3-PDH was commercialized by Biodesign Int. Cell culture Mouse neuroblastoma cell line (N2a) was purchased at the American Type Culture Collection (ATCC CCL131). N2a cells were stably transfected with wild-type mouse Prnp cDNA as decribed earlier ( Lehmann and Harris 1995). N2a58 subclone, overexpressing MoPrP proteins, was chronically infected with the mouse adapted scrapie strain 22L, as described by Nishida et al. ( Nishida et al. 2000). Non-infected N2a58 cell line and prion-infected N2a58/22L cell line were cultured in MEM medium with L-Glutamine supplemented with 10 % Fetal Calf Serum, 1% penicillin-streptomycin and 300 μg/ml geneticin. Cells were maintained at 37°C with 5% CO 2. Screening procedure of the antibodies N2a58/22L cells seeded in 6-well plates (5.10 5 cells/well) containing 3 ml of supplemented MEM medium, were incubated with 10 μg/ml of non-purified antibodies (ascitis) over 3 days. Cell lysates were prepared as previously described ( Perrier et al. 2000). The protein concentration of each sample was measured with the BCA reagent (Pierce). Samples of equal protein amounts and volumes were digested with 20 μg/ml of proteinase K at a ratio of 1:50 (protease to protein) for 1h at 37°C. Digestion were stopped by 1 mM Pefabloc and the samples were centrifuged at 20 000 g, for 30 min at 4°C. Pellets were resuspended in 40 μl of SDS Denaturation buffer, and boiled 3 min before loading them on 12 % SDS-PAGE precast Criterion gel (Biorad). Western blotting of separated proteins was performed by standard procedures. The MoPrP was detected by using a mixture of three monoclonal antibodies SAF60, SAF69 and SAF70, termed SAFmix, as previously described ( Nishida et al. 2000). Dose-dependent inhibition of PrPSc formation Prion-infected cells (about 10 6) plated in 25-cm 2 flasks were incubated with purified monoclonal antibodies either SAF34 or SAF61, at various concentrations, over 3 days. At confluence, cells were splitted at 1:4 dilution in a 25-cm 2 flask and incubated with their corresponding purified monoclonal antibody for another period of 3 days. After 6 days of treatment, cells were rinsed with cold PBS and lyzed with 500 μl of Lysis Buffer. Cells lysates were analyzed by immunoblotting according to standart procedures. The quantification of the PrP Sc was carried out by densitometric analysis of the films using the image analysis software Scion.Image Beta 4.02, Scion Corporation. Toxicity of anti-PrP antibodies onto the cells At confluence, cells were collected after trypsin treatment and resuspended in 4 ml of MEM medium. 0.2 ml of the cell suspension was mixed with 0.8 ml of a solution of 0.25 % of Trypan Blue (Sigma) and 10 μl of this mixture was distributed inside a numeration chamber (Kova glasstic slide). The non viable cells absorbed the dye and can thus be differentiated according to their blue coloration from the viable cells. The total number of viable and non viable cells, was counted in the different flasks treated or not with SAF34 and SAF61 antibodies. Time course inhibition assay in normal N2a58 and prion-infected N2a58/22L cell lines Identically seeded cells (about 10 6 cells in 25-cm 2 flasks) were incubated with 1 μg/ml of purified monoclonal antibodies either SAF34 or SAF61 or both of them. After 3 days, confluent cells were serially split in duplicate flasks, one flask used for western blot analysis and the other one for the prolongation of the treatment with the antibody. The treatment was performed for an extended period of time, up to 20 days. Every 3 days, cell lysates from N2a58/22L were collected and analyzed according to the protocol described above. Cellular extracts were also probed with Mab Glyceraldehyde-3-PDH at 0.5 μg/ml before proteinase K digestion, to check if the protein content was equivalent between each samples. Metabolic labeling and Immunoprecipitation of PrPC in N2a58 cells N2a58 cells at 80 % confluence in 35 mm dish were washed 3 times with PBS and incubated 1h with RPMI-1640 without methionine/cysteine containing 1% FCS. To analyze the effect of anti-PrP antibodies on PrP C half-life, cells were pulse-labeled (300 μCi/dish) for 1 hour and rinsed with MEM medium. During the chase, 2 μg/ml of SAF34 or SAF61 antibodies, or both of them at 2 μg/ml each, were added to the cells for a period of 1h, 3h and 5h. To analyze the effect of anti-PrP antibodies on PrP C synthesis, cells were pulse-labeled (300 μCi/dish) for 30 min and no chase was done. Then, cells were rinsed 3 times with 1 ml of cold PBS and lyzed by addition of 600 μl of lysis buffer (supplemented with 0.5% SDS). Samples were boiled 10 min at 95°C before immunoprecipitation of the PrP C. 200 μl of sample were diluted with 800 μl of buffer (0.5% Triton X-100, 50 mM TrisHCl pH 7.5, anti-proteases cocktail) before their incubation with 5 μl of purified SAF61 antibodies overnight at 4°C. Protein A sepharose beads were added for 2h at 4°C. The immuno-adsorbed proteins were washed 3 times in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM TrisHCl pH 7.5) and beads were resuspended in Laemmli buffer, then boiled for 10 min at 95°C. After centrifugation, immunoprecipitated proteins were collected in the supernatant and loaded on 12.5% SDS-PAGE gel, followed by autoradiography. mRNA analysis by RT-PCR Total RNA was extracted from antibody treated N2a58 cells (10 6 cells) using the SV Total RNA Isolation System (Promega). Then mRNA was isolated from total RNA using the PolyAtract mRNA Isolation System (Promega). Reverse transcription was performed on 1 μg of total RNA in 7 μl of water, 13 μl of polymerization mix (using 1X buffer, 1.5 mM dNTP, 1X RNasine, Reverse transcriptase MLV from Promega) and 1 μl of random primers (Gibco) after which the mixture was incubated for 2 hours at 37°C. The PCR was performed with 1 μl of reverse transcription mix, using the high fidelity long expand thermostable polymerase (Roche), 100 ng of oligonucleotides P1 ( 5′ ATGGCGAACCTTGGCTACTG 3′) and P2 ( 5′TCATCCCACGATCAGGAAGA 3′) corresponding to the 5′ and 3′ end of the PrP ORF, in a final volume of 50 μl. Thirty five amplification cycles were performed with an annealing temperature of 56°C. The PCR products were separated on agarose gel (1%). To analyze the relative expression of PrP mRNA, the signal from PrP mRNA was normalized based on the signal from ubiquitously expressed GAPDH mRNA. |
Screening of monoclonal antibodies for their capacity to inhibit PrPSc formation Seven antibodies corresponding to four different epitopes were screened for their potential anti-prion activity. The choice of the antibodies was based on their corresponding epitope, especially when the epitope is located in regions implicated in the misfolding of the PrP protein, as well as their ability to immunoprecipitate the PrP protein in cell lysates ( Table 1) ( Knaus et al. 2001; Peretz et al. 2001). Prion-infected cells (N2a58/22L) were incubated with non-purified antibodies at a concentration of 10 μg/ml for three days. At confluence, cells were lyzed and the lysates were submitted to a partial proteinase K digestion. The level of PrP Sc in the samples was analyzed by immunoblotting. Initial screening of antibodies identified four potential inhibitors of PrP Sc formation ( Figure 1A). To check the reproducibility of the inhibition and the dose-dependent effect, the four potential candidates were tested on cells at concentrations varying from 5 to 20 μg/ml. Only SAF34 and SAF61 resulted in a decrease of the PrP Sc levels that is reproducible and dose-dependent ( Figure 1B). SAF37 failed to exhibit a classical dose-dependent inhibition curve and was toxic for the cells at 20 μg/ml whereas the inhibition observed originally with 11C6 antibody was not reproducible ( Figure 1A and B). Results obtained with SAF60 and SAF70 antibodies suggest that their corresponding epitope (Tyr157 to Val161) is probably not implicated in the replication process of PrP Sc molecules or that the epitope is hindered in the native form of the PrP. We determined the antibody concentration at which 50% of the PrPSc levels are inhibited (IC50). N2a58/22L cells were incubated with various concentrations of purified antibodies for six days. The level of PrPSc in cells treated with purified SAF34 or SAF61 antibodies, compared with that of non-treated cells was dramatically reduced in a dose-dependent manner (Figure 2A and B). The IC50 values obtained for SAF34 and SAF61 were 1.3 ± 0.1 μg/ml (8.7 ± 0.7 nM) and 0.8 ± 0.05 μg/ml (5.3 ± 0.3 nM), respectively. In addition, the toxic effects of the antibodies on N2a58/22L cells, were assessed by performing a cell numeration using the Trypan Blue dye. The results did not show any differences between the non treated control cells and the cells treated either with SAF34 or SAF61 antibodies (data not shown). Time course inhibition of PrPSc with SAF34 and SAF61 antibodies To maximize the inhibitory effect, co-treatment experiments of cells with both antibodies were carried out. Cells treated simultaneously with SAF34 and SAF61 at 1 μg/ml each during three days, underwent a more potent inhibition of their PrP Sc levels than cells treated with only one of the two antibodies ( Figure 3A, lanes 1–5). Only 9 % of the PrP Sc molecules remain in the cells incubated with both SAF34 and SAF61, whereas 80 and 43 % of PrP Sc are detected in cells treated only with SAF34 and SAF61, respectively (Treated cells versus control untreated cells: P<0.001; ANOVA followed by Neuman-Keuls test) ( Table 2). In other words, the decrease of PrP Sc levels is 20% with SAF34, about 60 % with SAF61 and around 90 % with both antibodies. In pharmacological studies, additive effects are observed when the inhibition with 2 different molecules is equal to the sum of the inhibitions generated by each molecule alone. Synergistic effect appeared when the inhibitory effect is more potent than the additive effect. Additive effects can be observed only with molecules possessing independent binding sites and without saturating concentrations. SAF34 and SAF61 possess independent binding sites on the PrP molecule and demonstrated a compatible binding to PrP in sandwich ELISA experiments (J. Grassi, unpublished results). Thus, Table 2 shows the values expected if an additive effect was observed with both antibodies. Statistical analysis were done to determine whether the experimental values obtained after the co-treatment of cells with both SAF34 and SAF61 antibodies, are statistically significant from the values expected in the case of an additive effect (P <0.01, ANOVA followed by Neuman-Keuls test, see Table 2). Our results showed that the inhibitory effect observed after 3 days of co-treatment was synergistic. In order to see if we can completely cure the cells from their PrPSc molecules, the treatment was extended to 20 days, with 1 μg/ml of SAF34 or SAF61 or both antibodies (Figure 3A). After 6 days of incubation with the anti-PrP antibodies, results showed that PrPSc molecules remain only in cells treated with SAF34 antibodies. No PrPSc was detectable after 12 days of treatment. To address the long term efficiency of the treatment, cells that were previously incubated with the antibodies for 20 days, were then cultivated in their absence for another period of 20 days. Only the cells that had been pre-incubated with the SAF34 antibody, showed a reappearance of their PrPSc molecules after 6 days in absence of treatment (Figure 3B). The PrPSc levels slightly increased after 12, 16 and 20 days without SAF34 antibodies. Synergistic inhibition on PrPC levels in non infected cells Surprisingly, the antibodies decreased PrP C levels in uninfected cells and the effect was stronger in co-treatment experiments with SAF34 and SAF61 ( Figure 4A, lanes 1–5). After three days of treatment, 90 % and 67 % of PrP C levels were present in N2a58 cells treated with SAF34 and SAF61 respectively, as measured by densitometry analysis (Treated cells versus control untreated cells: P<0.001; ANOVA followed by Neuman-Keuls test) ( Table 2). By contrast, 33 % of the PrP C molecules remain in cells incubated with both antibodies. From these results, it was deduced that a decrease was obtained of 10% with SAF34, around 30 % with SAF61 and about 70 % with both antibodies. We calculated that about 60 % of the PrP C levels should remain if an additive effect was observed. As only 33 % of the PrP C levels are detectable, the inhibitory effect observed after three days of treatment with both antibodies is synergistic (co-treated cells versus expected values if additive effects: P<0.001, Table 2). Cell viability results showed that this effect was not associated with any toxicity arising from increasing concentrations of the antibodies (data not shown). Anti-PrP antibodies increase the degradation of the PrPC protein To evaluate the effect of the antibodies on PrP C degradation, we determined the half-life (T 1/2) of the PrP C protein in presence or absence of the antibodies. T 1/2 is the time required to clear 50% of the PrP C molecules. We carried out metabolic labeling experiments on uninfected N2a58 cells. Cultures were pulse-labeled during 1 hour, then the radioactive 35S medium was replaced by “cold” medium, followed by addition of 2 μg/ml of SAF34 or SAF61 or both antibodies during the chase period. T 1/2 of the PrP C protein was determined by densitometry analysis of the autoradiography. In the absence of antibodies, the T 1/2 for the PrP C protein was 5 hours ( Figure 5A and 5B), which is in agreement with previous studies ( Caughey et al. 1989; Borchelt et al. 1990; Borchelt et al. 1992). SAF61 antibodies diminished the T 1/2 for the PrP C protein from 5 to 3 hours and this dropped to 1.5 hours when both antibodies were present. By contrast, treatment with SAF34 antibodies did not significantly alter the T 1/2 of the PrP C protein (4.3 hours) ( Figure 5B). As a control it was identified that the antibodies did not act elsewhere other than the degradation of PrPC. To exclude the possibility that the antibodies inhibit PrPC during its biosynthesis, cells were pulse labeled in the presence of the antibodies and immediately lyzed without a chase period (Figure 5C). We chose a pulse of 30 min, which is the time required for exportation of the PrP protein to the membrane without degradation process. Equivalent amount of labeled PrPC was detected showing that antibodies alone or combined have no effect on PrPC biosynthesis (Figure 5C). We also verified that the antibodies do not decrease the PrP mRNA expression by performing RT-PCR analysis in N2a58 cells, previously incubated with both antibodies SAF34 and SAF61 at 1 μg/ml each (Figure 4B). Results showed that the PrP mRNA expression level is equivalent between the control not submitted to antibody treatment and the other sample treated with SAF34 and SAF61. The PrP mRNA expression levels in the cells were not modified by the antibodies. |
Because the large panel of antibodies generated by Grassi et al. (Demart et al. 1999) has never been tested for their potential anti-prion activity, a screening program was carried out using prion-infected cell cultures. We succeeded to find two inhibitory antibodies with different epitopes: SAF34 recognizing the flexible octarepeats region (59–89) and SAF61 which bound to the PrP amino acid residues (144–152). Comparison of SAF34 and SAF61 epitopes with those of antibodies that were previously screened for their anti-prion activity, was done by mapping them onto the PrP primary sequence (Figure 6). SAF34 is the only antibody directed against the N-terminal flexible region of the PrP that inhibits the PrPSc levels in the cells. As the SAF34 epitope recognized the octarepeat region, several antibodies might be bound to the repeated motifs (Table 1). Since E149 antibody directed against the last two octarepeat motifs (72–86) of PrP has no inhibitory effect on the PrPSc levels, we suggest that the first two octarepeat motifs (amino acid residues Q59-W72) are preferentially implicated in the inhibition process. In addition, SAF61 antibody recognized PrP residues (144–152) confirmed that the 132–156 region located in the core of the PrP protein is crucial for the generation of the PrPSc molecules. As D18 and SAF61 antibodies possessed similar epitopes on the PrP protein, it is interesting to compare their IC50 values (Figure 6). For SAF61, an IC50 of 5 nM was obtained, that is close to the value determined by Peretz et al., of 9 nM for the D18 antibody. Nevertheless, the duration of the treatment with D18 antibodies was 7 days compared to 6 days with SAF61. In both cases, antibody treatments were performed on prion-infected neuroblastoma cell lines, however, the N2a58/22L cells contained most likely a higher level of PrPSc than the ScN2a (Butler et al. 1988), since they are stably transfected with a murine Prnp gene (Nishida et al. 2000). Altogether these data may suggest that SAF61 is a more potent inhibitor of PrPSc formation than D18. In contrast, the binding of SAF60 and SAF70 onto the adjacent PrP residues (156–162) have no ability to block PrPSc formation. The mechanism leading to the disappearance of the PrPSc molecules remains to be elucidated. Two modes of action are possible: (i) antibodies act on PrPC or PrPSc isoforms; (ii) the antibodies prevent the formation of the molecular complexes between PrPC and PrPSc which is necessary for the replication process. SAF34 and SAF61 have a direct effect on the PrPC isoform. In the co-treatment experiments, we clearly showed that both antibodies remarkably decreased the PrPC levels in normal cells as well as in infected cells (data not shown). By shutting down almost 80 % of the PrPC levels, presumably the source for the generation of PrPSc, therefore the PrPSc levels are greatly affected. Concerning the mechanism inducing PrPC depletion, we can exclude the possibility that the antibodies interfered within the synthesis of PrPC since PrP mRNA expression remained unchanged either cells were treated or not with the antibodies. Since these results suggested that the antibodies were acting on PrPC degradation, metabolic labeling experiments were done to determine the half-life (T1/2) of the PrPC protein, with or without antibodies. We found a T1/2 for PrPC of 1.5 hours with both antibodies, compared to 5 hours in the control cells. Our data demonstrate that the degradation of the PrPC protein is markedly increased in the presence of both antibodies. As PrPC proteins are cleared much more rapidly, the consequence is that the PrPC levels are greatly lowered (23 % of PrPC was left at 6 days) in the cells. This mode of action is probably sufficient to completely eradicate the PrPSc in N2a58/22L cells. Experiments with normal cells treated with only one antibody showed that SAF34 and SAF61 antibodies have different mechanisms of action. The major mechanism of action of SAF61 is to increase the clearance of the PrPC. In the presence of SAF61 the T1/2 of PrPC was lowered to 3 hours, which provokes a strong diminution of the PrPC levels (43 % of PrPC was left at 6 days) in the cells. However it is probably not sufficient to completely eradicate PrPSc (less than 1% of PrPSc was left at 6 days), this would suggest the involvement of other mechanisms of action. As SAF61 antibodies recognized both PrP isoforms, it can be hypothesized that SAF61 also accelerates PrPSc degradation. By contrast to SAF61, SAF34 alone has a minor effect on the degradation of PrPC. The T1/2 of PrPC is nearly unchanged in presence of SAF34 (4.3 hours) that is in accordance with the almost unmodified PrPC levels (84 % of PrPC was left at 6 days) in the cells. From our results, we can conclude that SAF34 is not acting on PrPC degradation nor PrPC synthesis. In addition, the mode of action of the SAF34 antibody is not due to a direct effect on the PrPSc isoform, since this N-terminal antibody can not interact with the cleaved PrPSc which represents 95% of PrPSc in the ScN2a cells (Rachidi et al. 2003). The PrPSc inhibition by SAF34 antibody (35 % of PrPSc was left at 6 days) is likely due to the prevention of the formation of the molecular complexes between PrPC and PrPSc as suggested in previous studies (Enari et al. 2001; Peretz et al. 2001). The next step now is to understand how the degradation of PrPC by both antibodies occured. One striking feature is that the epitopes of SAF61 and SAF34 antibodies correspond to the two different binding sites (BD1 and BD2) of the laminin receptor precursor (LRP) on the PrP protein (Hundt et al. 2001). In the co-treatment experiments, one can hypothesizes that SAF61 might hide the direct binding site between the LRP and PrP (amino acids 144–179), while SAF34, might mask the interaction at the indirect binding site (BD2) between LRP and PrP via the heparan sulfate proteoglycan (amino acids 53–93). Since PrP interactions with its receptor are disrupted, it could lead to the destabilization of the PrP protein and its accelerated degradation. Indeed, the use of anti-LRP antibodies diminish both the PrPC as well as PrPSc levels in prion infected cells (Leucht et al. 2003). Alternatively, Mouillet-Richard et al (Mouillet-Richard et al. 2000) have suggested that the SAF61 antibody dimerizes the PrP proteins at the cell surface and induce signal transduction by phosphorylation of Fyn tyrosine kinase through caveolin intermediates. Moreover, we demonstrated that SAF61 antibodies are internalized in the cells (data not shown). It is possible that SAF61 induces a dimerization of PrP proteins, which might increase their internalization in the cells and activate the recycling of the protein in the endosomes. In other terms, SAF61 could modify the cellular trafficking of the prion protein. Numerous studies have showed that the binding of an antibody to a protein can lead to conformational changes inducing the release of cofactors, as well as the inhibition or activation of enzymatic catalysis (Crumpton 1966; Rotman and Celada 1968; Djavadi-Ohaniance et al. 1984). The binding of SAF61 to the PrP protein could induce conformational changes exposing amino acid residues of the PrP protein to proteases attacks. Another question concerns the inhibition of a putative function of the PrP protein by treatment with both antibodies. This gene is not lethal as demonstrated by the generation of mice Knock-Out for the Prnp gene which exhibit a normal development and behaviour (Bueler et al. 1992). Recently a conditional Knock-Out of the Prnp gene in adult mice was created and no effect on the animal viability was observed, suggesting that the loss of PrP function is not lethal for mice (Mallucci et al. 2002). To the contrary, the suppression of the PrP protein in neurons protected mice from neurodegeneration caused by prions (Mallucci et al. 2003). In this context, we may be confident that PrPC degradation caused by antibody treatment will not have a drastic effect on mice viability. The use of antibodies represents one of the most innovative therapeutic strategy for the treatment of the neurodegenerative disorders such as Alzheimer’s and Prion diseases diseases (Enari et al. 2001; Heppner et al. 2001; Peretz et al. 2001; White et al. 2003). However, such hopes were moderated since undesirable autoimmune reactions were observed in humans during clinical trials with the Aβ1-42 vaccine for Alzheimer’s disease (Birmingham et al., 2002). In the context of prion diseases, mice treated with high dose of anti-PrP antibodies ICSM18 and ICSM35 over an extended period of 53 weeks, did not show any evidences for autoimmune reaction (White et al. 2003). Because the treatment with SAF34 and SAF61 antibodies decrease the PrPC levels, it remains to determine whether an autoimmune reaction could appear during animal testing. Co-treatment experiments using antibodies possessing epitopes quite far from one another in the PrP sequence maximizes the inhibitory effect. Indeed, treatment with both SAF34 and SAF61 antibodies decreased the PrPC and the PrPSc levels synergistically suggesting that combining several antibodies in treatments would be a more efficient in vivo therapeutic strategy. Recently, antibodies that specifically target the PrPSc isoform were generated (Paramithiotis et al. 2003). As they presumably recognize the 162YYR164 PrPSc selective epitope, it would be interesting to test their effect ex-vivo and in vivo in a multi-treatment approach. Although serotherapy most certainly is of limited value once the clinical symptoms have manifested themselves (White et al. 2003), the immediate use of inhibitory antibodies as preventive treatment for people that were exposed to prions, after inadvertent manipulations of the infectious agent could be envisaged. |
We thank P. Rondard, O. Bischof, J.-L. Laplanche and J.-P. Pin for their fruitful discussions in the production of this manuscript. I am very grateful to S. Barrère for her assistance in the statistical analysis of the data. I am also grateful to H. Mc Mahon for her assistance in proofreading this manuscript. |
- Barret A, Tagliavini F, Forloni G, Bate C, Salmona M, Colombo L, De Luigi A, Limido L, Suardi S, Rossi G, Auvre F, Adjou KT, Sales N, Williams A, Lasmezas C, Deslys JP. Evaluation of quinacrine treatment for prion diseases. J Virol. 2003;77:8462–8469.
- Borchelt DR, Taraboulos A, Prusiner SB. Evidence for synthesis of scrapie prion proteins in the endocytic pathway. J Biol Chem. 1992;267:16188–16199.
- Borchelt DR, Scott M, Taraboulos A, Stahl N, Prusiner SB. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990;110:743–752.
- Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582.
- Butler DA, Scott MR, Bockman JM, Borchelt DR, Taraboulos A, Hsiao KK, Kingsbury DT, Prusiner SB. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. Journal of Virology. 1988;62:1558–1564.
- Caughey B, Race RE, Ernst D, Buchmeier MJ, Chesebro B. Prion protein biosynthesis in scrapie-infected and uninfected neuroblastoma cells. Journal of Virology. 1989;63:175–181.
- Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochem. 1991;30:7672–7680.
- Caughey WS, Raymond LD, Horiuchi M, Caughey B. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc Natl Acad Sci U S A. 1998;95:12117–12122.
- Collins SJ, Lewis V, Brazier M, Hill AF, Fletcher A, Masters CL. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann Neurol. 2002;52:503–506.
- Crumpton MJ. Conformational changes in sperm-whale metmyoglobin due to combination with antibodies to apomyoglobin. Biochem J. 1966;100:223–232.
- Demart S, Fournier JG, Creminon C, Frobert Y, Lamoury F, Marce D, Lasmezas C, Dormont D, Grassi J, Deslys JP. New insight into abnormal prion protein using monoclonal antibodies. Biochem Biophys Res Commun. 1999;265:652–657.
- Djavadi-Ohaniance L, Friguet B, Goldberg ME. Structural and functional influence of enzyme-antibody interactions: effects of eight different monoclonal antibodies on the enzymatic activity of Escherichia coli tryptophan synthase. Biochemistry. 1984;23:97–104.
- Donne DG, Viles JH, Groth D, Mehlhorn I, James TL, Cohen FE, Prusiner SB, Wright PE, Dyson HJ. Structure of the recombinant full-length hamster prion protein PrP(29–231): the N terminus is highly flexible. Proc Natl Acad Sci U S A. 1997;94:13452–13457.
- Enari M, Flechsig E, Weissmann C. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci U S A. 2001;98:9295–9299.
- Ghani AC, Ferguson NM, Donnelly CA, Hagenaars TJ, Anderson RM. Estimation of the number of people incubating variant CJD. Lancet. 1998;352:1353–1354.
- Heppner FL, Musahl C, Arrighi I, Klein MA, Rulicke T, Oesch B, Zinkernagel RM, Kalinke U, Aguzzi A. Prevention of scrapie pathogenesis by transgenic expression of anti- prion protein antibodies. Science. 2001;294:178–182.
- Hundt C, Peyrin JM, Haik S, Gauczynski S, Leucht C, Rieger R, Riley ML, Deslys JP, Dormont D, Lasmezas CI, Weiss S. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. Embo J. 2001;20:5876–5886.
- Knaus KJ, Morillas M, Swietnicki W, Malone M, Surewicz WK, Yee VC. Crystal structure of the human prion protein reveals a mechanism for oligomerization. Nat Struct Biol. 2001;8:770–774.
- Korth C, May BC, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci U S A. 2001;98:9836–9841.
- Krasemann S, Groschup M, Hunsmann G, Bodemer W. Induction of antibodies against human prion proteins (PrP) by DNA-mediated immunization of PrPO/0 mice. J Immunol Methods. 1996a;199:109–118.
- Krasemann S, Groschup MH, Harmeyer S, Hunsmann G, Bodemer W. Generation of monoclonal antibodies against human prion proteins in PrP0/0 mice. Mol Med. 1996b;2:725–734.
- Lehmann S, Harris DA. A mutant prion protein displays an aberrant membrane association when expressed in cultured cells. J Biol Chem. 1995;270:24589–24597.
- Leucht C, Simoneau S, Rey C, Vana K, Rieger R, Lasmezas CI, Weiss S. The 37 kDa/67 kDa laminin receptor is required for PrP(Sc) propagation in scrapie-infected neuronal cells. EMBO Rep. 2003;4:290–295.
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874.
- Mallucci GR, Ratte S, Asante EA, Linehan J, Gowland I, Jefferys JG, Collinge J. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. Embo J. 2002;2l:202–210.
- Meyer RK, McKinley MP, Bowman KA, Braunfeld MB, Barry RA, Prusiner SB. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986;83:2310–2314.
- Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–1928.
- Nishida N, Harris DA, Vilette D, Laude H, Frobert Y, Grassi J, Casanova D, Milhavet O, Lehmann S. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J Virol. 2000;74:320–325.
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A. 1993;90:10962–10966.
- Paramithiotis E, Pinard M, Lawton T, LaBoissiere S, Leathers VL, Zou WQ, Estey LA, Lamontagne J, Lehto MT, Kondejewski LH, Francoeur GP, Papadopoulos M, Haghighat A, Spatz SJ, Head M, Will R, Ironside J, O’Rourke K, Tonelli Q, Ledebur HC, Chakrabartty A, Cashman NR. A prion protein epitope selective for the pathologically misfolded conformation. Nat Med. 2003;9:893–899.
- Peretz D, Williamson RA, Kaneko K, Vergara J, Leclerc E, Schmitt-Ulms G, Mehlhorn IR, Legname G, Wormald MR, Rudd PM, Dwek RA, Burton DR, Prusiner SB. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature. 2001;412:739–743.
- Perrier V, Wallace AC, Kaneko K, Safar J, Prusiner SB, Cohen FE. Mimicking dominant negative inhibition of prion replication through structure-based drug design. Proc Natl Acad Sci U S A. 2000;97:6073–6078.
- Priola SA, Raines A, Caughey WS. Porphyrin and phthalocyanine antiscrapie compounds. Science. 2000;287:1503–1506.
- Prusiner SB, Scott MR, Dearmond SJ, Cohen FE. Prion protein biology. Cell. 1998;93:337–348.
- Rachidi W, Mange A, Senator A, Guiraud P, Riondel J, Benboubetra M, Favier A, Lehmann S. Prion infection impairs copper binding of cultured cells. J Biol Chem. 2003
- Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wuthrich K. NMR structure of the mouse prion protein domain PrP(121–321). Nature. 1996;382:180–182.
- Rotman MB, Celada F. Antibody-mediated activation of a defective b-D-galactosidase extracted from an Escherichia coli mutant. Biochemistry. 1968;60:660–667.
- Sethi S, Lipford G, Wagner H, Kretzschmar H. Postexposure prophylaxis against prion disease with a stimulator of innate immunity. Lancet. 2002;360:229–230.
- Supattapone S, Nguyen HO, Cohen FE, Prusiner SB, Scott MR. Elimination of prions by branched polyamines and implications for therapeutics. Proc Natl Acad Sci U S A. 1999;96:14529–14534.
- Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274:2079–2082.
- White AR, Enever P, Tayebi M, Mushens R, Linehan J, Brandner S, Anstee D, Collinge J, Hawke S. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature. 2003;422:80–83.
- Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofinan A, Smith PG. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;341:921–925.
- Wille H, Michelitsch MD, Guenebaut V, Supattapone S, Serban A, Cohen FE, Agard DA, Prusiner SB. Structural studies of the scrapie prion protein by electron crystallography. Proc Natl Acad Sci U S A. 2002;99:3563–3568.
|
 | Figure 1 Screening of monoclonal antibodies in prion-infected N2a58/22L cells |
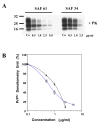 | Figure 2 Dose-dependent inhibition of PrPSc formation in N2a58/22L cells by purified monoclonal antibodies SAF34 and SAF61 |
 | Figure 3 Disappearance of PrPSc molecules from prion-infected N2a58/22L cells treated with the purified antibodies |
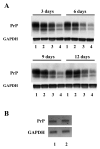 | Figure 4 Purified antibodies diminished the level of the PrPC molecules in non infected N2a58 cells |
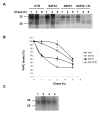 | Figure 5 Anti-PrP antibodies decreased the half-life of the PrPC protein |
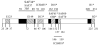 | Figure 6 Epitopes mapping of the various anti-PrP antibodies tested ex vivo or in vivo. |
 | Table 1 List of the monoclonal antibodies tested and their corresponding epitopes on the HuPrP protein sequence |
 | Table 2 Quantification of PrPC in normal N2a58 cells and PrPSc in prion infected N2a58/22L cells after 3 and 6 days of treatment with antibodies |
|

