Selection for intrabody solubility in mammalian cells using GFP fusions.
Résumé
Single-chain antibody fragments (scFv) expressed in the cytoplasm of mammalian cells, also called intrabodies, have many applications in functional proteomics. These applications are, however, limited by the aggregation-prone behaviour of many intrabodies. We show here that two scFv with highly homologous sequences and comparable soluble expression levels in Escherichia coli cytoplasm have different behaviours in mammalian cells. When over-expressed, one of the scFv aggregates in the cytoplasm whereas the second one is soluble and active. When expressed at low levels, using a retroviral vector, as a fusion with the green fluorescent protein (GFP) the former does not form aggregates and is degraded, resulting in weakly fluorescent cells, whereas the latter is expressed as a soluble protein, resulting in strongly fluorescent cells. These data suggest that the GFP signal can be used to evaluate the soluble expression of intrabodies in mammalian cells. When applied to a subset of an E.coli-optimised intrabody library, we showed that the population of GFP+ cells contains indeed soluble mammalian intrabodies. Altogether, our data demonstrate that the requirements for soluble intrabody expression are different in E.coli and mammalian cells, and that intrabody libraries can be directly optimised in human cells using a simple GFP-based assay.
Fichier principal
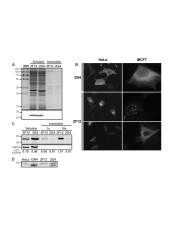 figure1.pdf (2.76 Mo)
Télécharger le fichier
Guglielmi.pdf (155.94 Ko)
Télécharger le fichier
figure2.pdf (262 Ko)
Télécharger le fichier
figure3.pdf (4.78 Mo)
Télécharger le fichier
figure4.pdf (41.66 Ko)
Télécharger le fichier
figure5.pdf (100.66 Ko)
Télécharger le fichier
inserm-00633286_edited.pdf (515.46 Ko)
Télécharger le fichier
supplementary_S1.pdf (333.13 Ko)
Télécharger le fichier
supplementary_S2.pdf (580.44 Ko)
Télécharger le fichier
supplementary_S3.pdf (425.63 Ko)
Télécharger le fichier
figure1.pdf (2.76 Mo)
Télécharger le fichier
Guglielmi.pdf (155.94 Ko)
Télécharger le fichier
figure2.pdf (262 Ko)
Télécharger le fichier
figure3.pdf (4.78 Mo)
Télécharger le fichier
figure4.pdf (41.66 Ko)
Télécharger le fichier
figure5.pdf (100.66 Ko)
Télécharger le fichier
inserm-00633286_edited.pdf (515.46 Ko)
Télécharger le fichier
supplementary_S1.pdf (333.13 Ko)
Télécharger le fichier
supplementary_S2.pdf (580.44 Ko)
Télécharger le fichier
supplementary_S3.pdf (425.63 Ko)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Origine : Fichiers produits par l'(les) auteur(s)
Format : Autre
Format : Autre
Format : Autre