| |
| J Infect Dis. 2009 January; 199(1): 14–9. doi: 10.1086/595566.Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in Orange Farm, South Africa Bertran Auvert,1* Joelle Sobngwi-Tambekou,2 Ewalde Cutler,3 Marthi Nieuwoudt,3 Pascale Lissouba,2 Adrian Puren,3 and Dirk Taljaard4 1Hôpital Ambroise Paré
AP-HP, Hôpital Ambroise Paré, Boulogne-Billancourt, F-92100,FR 2Santé publique et épidémiologie des déterminants professionnels et sociaux de la santé
INSERM : U687, IFR69, Université Paris Sud - Paris XI, Université de Versailles-Saint Quentin en Yvelines, Hôpital Paul Brousse 16, av Paul Vaillant Couturier 94807 VILLEJUIF,FR 3National Institute for Communicable Disease
National Institute for Communicable Disease, Johannesburg,ZA 4Progressus
Progressus, Johannesburg,ZA |
Introduction A causal association links high-risk human papillomavirus (HR-HPV) and cervical cancer, which is a major public health problem. The objective of this study was to investigate the association between male circumcision (MC) and HR-HPV prevalence among young men. Methods We used data from a male circumcision trial conducted in Orange Farm (South Africa) among men aged 18 to 24. Urethral swabs were collected during a period of 262 consecutive days among participants from the intervention (circumcised) and control (uncircumcised) groups who were reporting for a scheduled follow-up visit. Swabs were analyzed using PCR. HR-HPV prevalence rate ratios (PRRs) were assessed using univariate and multivariate log-Poisson regression. Results In an intention-to-treat analysis, HR-HPV prevalences among intervention and control groups were 14.8% (94/637) and 22.3% (140/627), respectively, with a PRR of 0.66 (0.51–0.86) P=0.002. Controlling for propensity score and confounders (ethnic group, age, education, sexual behavior including condom use, marital status, and HIV status) had no effect on the results. Conclusions This is the first randomized controlled trial that shows a reduction in urethral HR-HPV infection following male circumcision. This finding explains why women with circumcised partners are less at risk of cervical cancer than other women. MESH keywords: Adolescent, Circumcision, Male, statistics & numerical data, Gonorrhea, epidemiology, HIV Infections, epidemiology, Humans, Male, Neisseria gonorrhoeae, isolation & purification, Papillomavirus Infections, epidemiology, Prevalence, Risk Assessment, Risk Factors, Sexual Behavior, physiology, South Africa, epidemiology, Urethra, virology, Young Adult Author keywords: HPV, male circumcision, Africa, randomized controlled trial |
A recent meta-analysis estimated worldwide human papillomavirus (HPV) prevalence among women at 10.4% [1]. HPV genotypes are divided into “high-risk” and “low-risk” genotypes, on the basis of their association with cervical lesions. The high-risk human papillomavirus (HR-HPV) types are more frequently found in pre-malignant or malignant lesions and are associated with cancers of the cervix, vulva, vagina, anus, and penis [2–4]. A causal association between cervical cancer and HR-HPV has now been established [4–9] and worldwide HR-HPV prevalence of cervical carcinomas has been estimated at 99.7% [10]. Cervical cancer is the most common cancer affecting women in developing countries, with over 70% of cases occurring in Africa being attributed to HR-HPV genotypes 16 and 18 [1, 11, 12]. Thus, any factor reducing the probability of acquiring or transmitting HPV will also considerably reduce the burden of disease, especially in the developing world [4]. Observational studies have suggested that HPV prevalence is reduced among circumcised men compared with uncircumcised men [3, 4, 13–15]. Nevertheless, such an association has not yet been proven using a randomized controlled trial (RCT). The objective of this study was to analyze the effect of male circumcision (MC) on HR-HPV prevalence using data collected during a male circumcision RCT conducted in Orange Farm (South Africa), which demonstrated a partial protective effect of MC on the acquisition of HIV by young males. |
Collection of data The technical details of the trial (ANRS-1265) have been published elsewhere [ 16] and only a summary will be presented in this article. Between February 2002 and July 2004, 3274 uncircumcised males, aged 18 to 24, were recruited, randomized into two groups and followed up. MC was offered immediately after randomization to the intervention group and after the end of the follow-up period to control group participants. During each follow-up visit at 3, 12 and 21 months, circumcision status was assessed by a nurse through genital examination. In addition, information about sexual behavior was collected, including number of partners as a function of time, number of sexual contacts with each partner, condom use and age of partners. During 262 consecutive days, from March 7th, 2005 to November 24th, 2005, a urethral swab was collected by a nurse from all participants coming for the 21-month visit. All participants signed a written consent form for this test to be performed. Due to limited funding, the collection of swabs for HR-HPV testing was not started earlier. The urethra was chosen because the detection of HPV in this anatomical site is probably not affected by circumcision status. These swabs were analyzed to assess the association between the prevalence of HR-HPV strains and male circumcision. A urethral swab was also collected at a follow-up visit about 6 weeks after MC from all control group participants who took part in a nested study designed to compare two circumcision methods. To ascertain that the detection of HR-HPV was not affected by circumcision status, we used these swabs to compare the prevalence of HR-HPV among the nested study participants before and after circumcision. Lastly, study participants were asked to give a first-void urine sample in order to test for urogenital Neisseria gonorrhea (NG), which presence was used as a biological marker of sexual behavior. Laboratory methods Specimens were frozen at −20°C immediately after collection and kept frozen until processing. DNA was extracted from the urethral swabs using the MagNA Pure LC (Roche) instrument, with the Roche MagNA Pure LC DNA I Isolation Kit. Swabs were lysed in 500 μl of the kit lysis buffer for 30 minutes at room temperature. The MagNa Pure external lysis protocol was used to extract DNA from the lysis buffer into a 100 μl eluate. 50 μl of the eluate was used for screening (Roche Amplicor HPV test) and 50 μl eluate was used for genotyping (Roche Linear Array Genotyping test). This standardized PCR-based method can detect 13 HR genotypes of HPV (i.e., genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). Because of the combined probe of the assay for HPV-52 and in order to be conservative, samples were classified as HPV-52 positive only when they were negative for genotypes 33, 35 and 58. Negative results with a negative internal beta globin PCR control were excluded. All positives were genotyped. An HPV-positive sample was defined by a sample where at least one HR-HPV was detected. In some analyses we also considered multiple HPV samples defined for samples where at least two HR-HPV genotypes were detected. Urine specimens were tested for NG by PCR (Roche Cobas Amplicor PCR). Data analysis The intention-to-treat and as-treated prevalence rate ratios (PRRs) of HR-HPV-positivity and NG-positivity were estimated using univariate log-Poisson regression These analyses were repeated multivariately by controlling for ethnic group, age, education, number of lifetime partners, marital status, number of non-spousal partners in the past 12 months, condom use in the past 12 months, number of sex acts in the past 12 months and HIV status. To assess the potential impact of HIV acquisition, which is reduced by MC and is associated with HPV infection [17], these analyses were repeated after excluding those who seroconverted for HIV during the follow-up period (n=25). To evaluate a possible imbalance between the groups, analyses were repeated when controlling for propensity score coded in quintiles [18]. Statistical analyses were performed using the statistical package SPSS for Windows version 8 (SPSS, Chicago, Illinois, United States) and R programming language (version 2.6.1) [19]. Ethics The research protocol was reviewed and approved by the University of Witwatersrand Human Research Ethics Committee (Medical) on February 22 nd, 2002 (protocol study no. M020104). The trial was also approved by the Scientific Commission of the French National Agency for AIDS Research (ANRS; protocol study no. 1265; 2002, decision No. 50) and authorization was obtained from the City of Johannesburg, Region 11, on 25 February 2002. This trial has been registered at http://www.clinicaltrials.gov under the number NCT00122525. |
Baseline characteristics of the 1264 participants from whom a urethral swab was collected at the 21-month visit are reported by randomization group in Table 1. These characteristics are similar and not statistically different except for HIV status. The mean (median) duration in days of follow-up among the intervention and control group was 644 (637) vs. 649 (637), respectively. Table 2 presents the intention-to-treat univariate association between HR-HPV prevalence and male circumcision at the scheduled 21-month visit. HR-HPV prevalence was significantly lower among men of the intervention group. As indicated in Figure 1, the percentage of each of the 13 HR-HPV genotypes was always lower among men of the intervention group than among men of the control group. In the intention-to-treat comparison, the difference was significant for genotypes 18, 31, 45, 52, 56, 58, and 68. Table 2 shows that the protective effect of MC on HR-HPV is higher in the as-treated analysis than in the intention-to-treat analysis. The protective effect is also higher in both analyses when controlling for potential confounders, including HIV status and reported sexual behavior cofactors. HR-HPV was associated with HIV status in both analyses with aPRRs of 2.2 (95%CI 1.5–3.3) and 2.2 (95%CI 1.5–3.2), respectively. When those who seroconverted for HIV during follow-up were excluded from the analysis, the results indicated in Table 2 remained practically unchanged with P-values less than 0.009 and a relative variation of the PRRs and aPRRs of less than 5.2%. This suggests that the effect of MC on HR-HPV is independent of the effect of MC on HIV. The aPRRs were almost identical when the analyses were adjusted for the propensity score in addition to the other covariates. Multiple HR-HPV prevalence was 7.0% (89/1267; 95% CI: 5.7% – 8.6%). It was significantly lower among men of the intervention group compared with men of the control group (4.2% vs. 9.9%; PRR=0.43; 95% CI: 0.28 – 0.66; P<0.001). Among men with at least one HR-HPV, multiple HR-HPV prevalence was also lower among men of the intervention group (44.3% vs. 28.7%; PRR=0.64; 95% CI: 0.45 – 0.94; P=0.020). As indicated in Table 3, NG prevalence was similar in the two groups. Among men of the control and intervention groups, median number of lifetime partners was 4.1 and 4.2, respectively, (P=0.49; Kruskal-Wallis test) and the proportion of consistent condom users was 17.4% and 19.7%, respectively, (P=0.45; Fisher exact test). These findings suggest that the protective effect of MC on HR-HPV cannot be attributed to a difference of sexual behavior between the two groups. During the study period, 371 men of the control group were circumcised and had a urethral swab taken before and after male circumcision. The average (median) duration between the two swab collections was 59 (43) days. As expected, the HR-HPV prevalence was the same between the two samplings (23.7% vs. 23.9%; P=1.0; Sign test). The proportion of males with multiple HR-HPV genotype infections was not significantly different (10.2% vs. 12.1%; P=0.40; Sign test). These results indicate that the as-treated effect of male circumcision on HR-HPV prevalence shown in Table 2 cannot be attributed to an easier detection of HR-HPV by urethral swabbing in uncircumcised men compared with circumcised men. |
Using data collected during the MC trial conducted in Orange Farm (South Africa), we demonstrated an independent and partial protective effect of male circumcision on HR-HPV prevalence. The effect was shown on HR-HPV prevalence and not incidence due to the available biological samples in this MC trial. This effect remained unchanged when the analysis was adjusted for possible confounding factors such as sexual behavior and condom use. Due to a) the randomization, b) the results of the propensity analysis, and c) the absence of obvious differences in the gonorrheal prevalence and sexual behavioral characteristics between the intervention and control groups, the HR-HPV prevalence difference between the two groups is likely attributable to male circumcision. In this view, the difference observed is probably the consequence of a difference in HR-HPV incidence between circumcised and uncircumcised men. Indeed, in this study HR-HPV prevalence is likely a proxy for HR-HPV incidence because among young men HPV prevalence is rising as a function of age [20]. This study has some limitations. Biological samples were not collected throughout the follow-up period, so the HR-HPV status at inclusion is unknown. This information would have allowed us to compare HR-HPV incidence as a function of MC status and HR-HPV prevalence between intervention groups at inclusion. Because some participants were certainly already infected by HR-HPV at inclusion, the effect measured on prevalence is an underestimation of the true effect of MC. Secondly, the fact that participants were not blind to the intervention could have led to sexual behavior change and bias. Lastly, HR-HPV detection was performed on urethral swabs which are likely to miss infections [21]. Thus, the prevalence of HR-HPV infections in our cohort is likely to be underestimated since detection in the urethra is significantly lower than detection in the glans, corona sulcus or the penis shaft [21, 22]. However, we believe that there is no risk of non-differential misclassification since we did not find any difference when we compared the urethral HR-HPV prevalences before and after circumcision among a sub-sample of participants. Hence we believe that HR-HPV infections will be underestimated equally among the two arms and will have no effect on PRRs. Despite this loss of power, our study evidenced a significant protective effect of MC against HPV infection. In this study we could not detect an effect of MC on some HR-HPV genotypes such as genotypes 16 and 33. The apparent variation of the effect of MC according to genotype can be due to a true variation of the effect of MC according to genotypes or to random variation. This possible variation of the effect of MC according to genotypes should be further investigated for example by combining the results of this study with the results of the other MC trials conducted in Kenya and Uganda [23, 24]. The protective effect has a magnitude corresponding to what could have been expected from observational studies. Castellsague and colleagues reported in their meta-analysis an odds ratio of 0.56 (95% CI: 0.39–0.82) [13], while Baldwin and colleagues found an aRR of 0.44 (95% CI: 0.23–0.81) [3]. Similarly, Hernandez and colleagues found that uncircumcised men had nearly a two-fold (RR=1.96; 95% CI: 1.02–3.75) increased risk of oncogenic HPV infections [14]. Based on the results of the RTC, there is now clear evidence that male circumcision decreases the heterosexual risk of HR-HPV acquisition by males. HR-HPV is a major public health problem because of its causal association with malignancies, especially cervical cancer in women. Hence this study illustrates why MC has long been thought to be protective against cervical cancer [9]. Indeed, as shown in our study, MC reduced the risk of HR-HPV infection among men and consequently reduced the exposure of women to HR-HPV. Thus, the risk of cervical cancer is lowered because of the causal link between HR-HPV and cervical cancer among women [4–9]. Since three randomized controlled trials have shown a partial protective effect of MC on the acquisition of HIV by males in Africa [16, 23, 24], the effect of MC on HR-HPV reinforces the WHO-UNAIDS recommendation for the implementation of MC programs in countries with a high HIV prevalence, a low MC prevalence and a high MC acceptability [25]. These countries, mainly in Southern and Eastern Africa, are those where the affordability of the HPV vaccine remains a problem. Moreover, the protective effect of MC may supplant HPV vaccines in terms of genotype coverage and target group age-range. |
The authors would like to thank all those who agreed to take part in this study, answer the questions put to them, and provide swab samples. The authors would like to thank Gaph Sipho Phatedi for his management of the recruitment process and Yvon de La Soudière for his help in the management of the data set. The authors would like to thank the general practitioners who performed the male circumcisions for the study (Dr Bhekuyise Gwala, Dr George Shilaluke and Dr Dumiso Zulu). The authors would like to thank Goliath Gumede for the clinical investigation and Zodwa Nkosi for interviewing all the respondents. They would also like to thank Bongiwe Klaas for the data capture, Mabel Hunter and the recruitment staff and all the assistants (Cynthia Dlamini; Sidwell Dumisi; Benjamin Masitenyane; Robert Matodzi; Tsietsi Mbuso; Anthony Motha; Sibongiseni Mpetsheni; Jabulani Nhlapo; Joseph Ntsele; Male Chakela; Audrey Tshabalala; Donald Mashamba; Nkululeko Nhlapo) for their cooperation and support. Lesley Short, Moses Mashiloane, Beulah Miller, Beverley Singh, Sarah Hloma and the HIV serology laboratory of the National Institute for Communicable Diseases, Johannesburg, South Africa provided technical assistance in regard to the laboratory testing and administration.
Financial support
ANRS grant 1265 (France), Gates Foundation (USA) grant 33759, NICD (South Africa), INSERM (France). |
Footnotes |
1. de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. 2. Human papillomavirus and HPV vaccines: Technical information for policy-makers and health professionals. 2007 3. Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, Vaught LC, Giuliano AR. Condom use and other factors affecting penile human papillomavirus detection in men attending a sexually transmitted disease clinic. Sex Transm Dis. 2004;31:601–7. 4. Castellsague X, Bosch FX, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–12. 5. Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. 6. Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992;327:1272–8. 7. Lehtinen M, Luukkaala T, Wallin KL, et al. Human papillomavirus infection, risk for subsequent development of cervical neoplasia and associated population attributable fraction. J Clin Virol. 2001;22:117–24. 8. Rozendaal L, Westerga J, van der Linden JC, et al. PCR based high risk HPV testing is superior to neural network based screening for predicting incident CIN III in women with normal cytology and borderline changes. J Clin Pathol. 2000;53:606–11. 9. Morris BJ. Why circumcision is a biomedical imperative for the 21(st) century. Bioessays. 2007;29:1147–58. 10. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. 11. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. 12. Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–85. 13. Castellsague X, Albero G, Cleries R, Bosch FX. HPV and circumcision: a biased, inaccurate and misleading meta-analysis. J Infect. 2007;55:91–3. 14. Hernandez BY, Wilkens LR, Zhu X, et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J Infect Dis. 2008;197:787–94. 15. Vaccarella S, Lazcano-Ponce E, Castro-Garduno JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–9. 16. Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. 17. Ng’ayo MO, Bukusi E, Rowhani-Rahbar A, et al. Epidemiology of human papillomavirus infection among fishermen along Lake Victoria Shore in the Kisumu District, Kenya. Sex Transm Infect. 2008;84:62–6. 18. Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. 19. Team RDC. R Foundation for Statistical Computing. Vienna, Austria: 2005. R: A language and environment for statistical computing. 20. Okesola AO, Fawole OI. Prevalence of human papilloma virus genital infections in sexually transmitted diseases clinic attendees in Ibadan. West Afr J Med. 2000;19:195–9. 21. Giuliano AR, Nielson CM, Flores R, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis. 2007;196:1146–52. 22. Aguilar LV, Lazcano-Ponce E, Vaccarella S, et al. Human papillomavirus in men: comparison of different genital sites. Sex Transm Infect. 2006;82:31–3. 23. Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. 24. Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. 25. Policy and Programme Implications. UNAIDS-WHO; Geneva, Switzerland: 2007. New Data on Male Circumcision and HIV Prevention. |
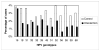 | Figure 1 Distribution of the high-risk HPV genotypes as a function of randomization group. |
 | Table 1 Background characteristics, reported sexual behavior and HIV prevalence at the 21-month visit |
 | Table 2 Association between High-risk Human Papillomavirus (HR-HPV) prevalence and male circumcision. |
 | Table 3 Association between Neisseria gonorrhea (NG) prevalence and male circumcision. |
|

